Review Article - (2025) Volume 9, Issue 1
Current Evidence On GLP-1 Receptor Agonists: Impact On Residual Gastric Content and Endoscopy Quality
2Division of Gastroenterology, East Tennessee State University, Johnson City, TN, USA
Received Date: Nov 18, 2024 / Accepted Date: Dec 30, 2024 / Published Date: Jan 22, 2025
Copyright: ©©2025 McKenna Andrews, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Andrews, M., Magacha, H., Berry, D., Nagpal, S., Young, M. F., et al. (2025). Current Evidence On GLP-1 Receptor Agonists: Impact On Residual Gastric Content And Endoscopy Quality. J Gastro & Digestive Systems, 9(1), 01-06.
Abstract
Glucagon-like peptide-1 (GLP-1) receptor agonists, introduced in 2005, have become crucial in managing type 2 diabetes mellitus (T2DM) by regulating gastrointestinal motility and metabolic disorders. These agents, including exenatide and liraglutide, effectively lower HbA1c levels, reduce body weight, and minimize hypoglycemia risk by mimicking the endogenous incretin hormone GLP-1. GLP-1 receptor agonists enhance insulin secretion, inhibit glucagon release, and promote satiety, which aids in glucose homeostasis and weight management. Additionally, these agonists provide cardiovascular benefits, reducing the risk of major adverse cardiovascular events in T2DM patients. The primary mechanism of action involves delaying gastric emptying by modulating smooth muscle contractions and inhibiting gastric secretions, thus helping flatten postprandial glucose spikes and enhance satiety. This delay in gastric emptying has significant clinical implications, especially during endoscopic procedures, as it increases residual gastric contents, potentially complicating visualization and increasing the risk of aspiration. Studies have shown that patients on GLP-1 receptor agonists exhibit higher residual gastric volumes, necessitating adjustments in fasting guidelines before endoscopy. Additionally, GLP-1 receptor agonists may affect bowel preparation quality during colonoscopy, leading to suboptimal visualization and increased procedural complications. Consequently, clinicians are advised to consider protocol adjustments, such as extended fasting periods and alternative bowel preparation regimens, to optimize endoscopic outcomes and minimize risks in patients on GLP-1 therapy. These findings underscore the need for individualized patient management and careful assessment of GLP-1 receptor agonist use in clinical practice.
Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists became available in 2005 as a significant therapeutic class with multifaceted roles in the regulation of gastrointestinal motility and metabolic disorders, particularly type 2 diabetes mellitus (T2DM) [1]. The use of GLP-1 receptor agonists, such as exenatide and liraglutide, has been shown to significantly lower hemoglobin A1c (HbA1c) levels, reduce body weight, and lower the risk of hypoglycemia compared to traditional insulin therapy. These agonists mimic the action of the endogenous incretin hormone GLP-1, which is secreted by the L-cells of the intestine in response to food intake [2]. GLP-1 plays a critical role in maintaining glucose homeostasis by enhancing insulin secretion in a glucose-dependent manner, inhibiting glucagon release, and promoting satiety. The exogenous administration of GLP-1 receptor agonists extends these benefits, providing a powerful tool for managing hyperglycemia and aiding weight loss in individuals with T2DM [3]. Additionally, GLP-1 receptor agonists have cardiovascular benefits, including reduced risk of major adverse cardiovascular events in patients with T2DM and established cardiovascular disease.
One of the primary mechanisms through which GLP-1 receptor agonists influence gastrointestinal motility is by delaying gastric emptying [4]. This delay is a result of the modulation of smooth muscle contractions in the stomach and the inhibition of gastric secretions. By slowing the rate at which food exits the stomach and enters the small intestine, GLP-1 receptor agonists help to flatten postprandial blood glucose spikes, which are critical for effective diabetes management [5]. This prolonged gastric emptying not only aids in blood glucose control but also enhances feelings of satiety, leading to reduced caloric intake and weight loss. For individuals with T2DM, who often struggle with obesity and insulin resistance, this dual effect is particularly beneficial, contributing to improved overall metabolic health [4,5]. Beyond gastric emptying, GLP-1 receptor agonists also affect other aspects of gastrointestinal motility, including small intestinal transit and colonic motility. In the small intestine, GLP-1 receptor activation leads to enhanced motility, facilitating the timely movement of chyme and ensuring efficient nutrient absorption. This effect is mediated through both the enteric nervous system and central nervous mechanisms, where GLP-1 receptors are expressed. The activation of these receptors results in a coordinated increase in peristaltic activity, which helps to maintain a steady progression of contents through the intestinal tract [6,7].
In the colon, GLP-1 receptor agonists modulate peristalsis, promoting a more coordinated and effective movement of fecal matter. This regulation of colonic motility is particularly important in preventing disorders associated with dysmotility, such as irritable bowel syndrome (IBS) and chronic constipation [8]. By enhancing coordinated contractions in the colon, GLP-1 receptor agonists can help maintain regular bowel movements and prevent the discomfort and complications associated with these conditions. The therapeutic potential of GLP-1 receptor agonists extends beyond their role in diabetes management and gastrointestinal motility regulation. Emerging research suggests their utility in treating a range of gastrointestinal disorders characterized by impaired motility, such as gastroparesis, a condition marked by delayed gastric emptying, and chronic constipation [8,9]. In patients with gastroparesis, GLP-1 receptor agonists can improve gastric motility and alleviate symptoms such as nausea, vomiting, and bloating. Similarly, for individuals with chronic constipation, these agents can enhance colonic peristalsis and promote regular bowel movements, offering a new avenue for treatment [10]. Furthermore, the impact of GLP-1 receptor agonists on gastrointestinal motility has implications for other aspects of health. For example, the modulation of gut motility and hormone release can influence gut-brain signaling, potentially affecting mood and cognitive functions. The interplay between the gut and the brain, often referred to as the gut-brain axis, is an area of active research, and GLP-1 receptor agonists are increasingly recognized for their potential to modulate this complex system [9-11].
Review of the Literature
Impact on Residual Gastric Contents During Endoscopy
The effect of GLP-1 on residual gastric contents during endoscopy is a specific area of interest. Endoscopic procedures often require the stomach to be empty to improve visualization and reduce complications. However, patients on GLP-1 receptor agonists may experience increased residual gastric contents due to delayed gastric emptying [8,12]. GLP-1 is known to influence gastric motility. It has been observed to slow gastric emptying, which can be beneficial in the management of type 2 diabetes mellitus (T2DM) and obesity, as it promotes satiety and reduces postprandial glucose spikes (1). Studies have demonstrated that GLP- 1 analogs, such as exenatide and liraglutide, delay gastric emptying by affecting the pyloric sphincter and reducing gastric motility [12,13]. Nauck et al., conducted a study comparing the effects of liraglutide and placebo on gastric emptying in diabetic patients. They found that liraglutide significantly delayed gastric emptying, which correlated with a reduction in postprandial glucose levels [14]. Similarly, Müller et al. (2017), reported that GLP-1 receptor agonists reduced gastric emptying rates and food intake in obese individuals, suggesting that these effects are consistent across different patient populations [12].
A study by Garza et al., explored the frequency of gastric residue on upper endoscopy in patients on GLP-1 receptor agonists and propensity score-matched controls in this retrospective case- control study over 3.5 years. They found that patients receiving GLP-1 therapy had significantly higher residual gastric volumes compared to those not on the medication. The study showed that the rate of gastric residue was higher (14% vs 4%, P < 0.01) in insulin dependent type 2 diabetic patients as well as in type 2 diabetic patients with complications (15% vs 2%, P < 0.01). This effect was attributed to the delayed gastric emptying induced by GLP-1 receptor agonists, which can lead to increased residual contents and potential complications during endoscopic procedures [14]. Sudipta et al., assessed the association between GLP-1 use and the prevalence of increased residual gastric contents as a major risk factor for aspiration during a procedure under anesthesia.
The study found that the prevalence of increased residual gastric contents was 56% (35 of 62) of patients on GLP-1 compared with 19% (12 of 62) of patients on control group. GLP-1 receptor agonists use was associated with a 30.5% (95% CI, 9.9%-51.2%) higher prevalence of increased RGC (adjusted prevalence ratio, 2.48; 95% CI, 1.23-4.97), (4). Another study done by Stark et al. (2021) retained food contents during upper endoscopy was documented in 6.8% of patients on GLP-1 versus 1.7% of patients in the control group (odds ratio [OR] 4.22 [95% CI 0.87-20.34]), [15,16]. In a study by Fei et al. (2024) the researchers investigated the impact of GLP-1 receptor agonists on residual gastric contents in patients who were fasting prior to endoscopic procedures under anesthesia. The study found that patients on GLP-1 receptor agonist therapy had a significantly higher incidence of residual gastric contents compared to those not receiving these medications. Residual gastric contents were documented in 17 procedures (19%) in the GLP group versus 5 procedures (5%) in the control group with an associated confounder adjusted odds ratio of 5.8 (95% confidence interval, 1.7 to 19.3; P = 0.004) [17]. Another study by Chiu et al., evaluated the practical implications of GLP-1 use in patients undergoing elective endoscopic procedures. They reported that the presence of residual gastric contents could interfere with the endoscopic procedure, necessitating adjustments in pre-procedure fasting guidelines for patients on GLP-1 therapy. The study recommended that healthcare providers consider extending the fasting period or adjusting the timing of GLP-1 administration before endoscopy to mitigate this issue [18].
Clinical Implications
The delayed gastric emptying associated with GLP-1 receptor agonists has significant implications for endoscopic procedures. Residual gastric contents can obscure visualization and increase the risk of aspiration or other procedural complications. Clinicians need to be aware of this effect when managing patients on GLP-1 therapy, particularly in preparation for endoscopic examinations.
Quality of Bowel Preparation
The quality of endoscopy, a critical diagnostic and therapeutic procedure for gastrointestinal (GI) diseases, can be influenced by various factors, including the use of GLP-1 receptor agonists. High- quality endoscopy ensures accurate diagnosis, effective treatment, and improved patient outcomes. For patients with T2DM using GLP-1 receptor agonists, several aspects of endoscopy quality are particularly relevant:
• Gastric Emptying and Preparation: GLP-1 receptor agonists delay gastric emptying, which can affect the preparation for upper GI endoscopy. Adequate fasting times must be ensured to prevent residual food in the stomach, which can obscure visualization and complicate the procedure.
• Detection of GI Complications: The delayed gastric emptying and altered GI motility associated with GLP-1 receptor agonists can influence the presentation of GI symptoms and complications. Endoscopists need to be aware of these effects to accurately interpret findings and avoid misdiagnosis. For instance, the presence of residual food or delayed transit time might necessitate a repeat endoscopy or additional imaging studies.
• Therapeutic Interventions: High-quality endoscopy also involves effective therapeutic interventions, such as removing polyp or treating GI bleeding. In patients using GLP-1 receptor agonists, the modulation of GI motility and hormone release can impact the response to these interventions. Ensuring thorough documentation and follow-up is crucial for assessing treatment efficacy and patient safety.
• Safety and Complications: The safety profile of endoscopic procedures in patients using GLP-1 receptor agonists must be carefully managed. Potential complications related to altered GI motility, such as gastroparesis or constipation, require vigilant monitoring and tailored endoscopic techniques to minimize risks and optimize outcomes. Comparatively, non- GLP-1 users might not present the same challenges related to delayed gastric emptying and altered GI motility, potentially leading to more straightforward endoscopic procedures. However, the comprehensive management of diabetes and associated complications in these patients remains equally important to ensure high-quality endoscopy and optimal patient care.
Bowel preparation quality is a critical factor in the effectiveness of colonoscopy, as it directly impacts visualization, diagnosis, and procedural outcomes. GLP-1 receptor agonists, a class of medications commonly used in the management of type 2 diabetes, have been shown to alter gastrointestinal transit and motility. These changes may influence the effectiveness of bowel preparation. Several studies have evaluated the impact of GLP- 1 receptor agonists on bowel preparation quality and potential adjustments needed for patients on these medications. The study by Morris et al. (2021) examined the quality of bowel preparation in patients using GLP-1 receptor agonists. The researchers conducted a prospective study involving 300 patients scheduled for colonoscopy, with 150 patients on GLP-1 receptor agonists and 150 patients not on these medications. The primary endpoint was the quality of bowel preparation, assessed using the Boston Bowel Preparation Scale (BBPS). The study found that patients on GLP-1
receptor agonists had significantly lower BBPS scores compared to those not on these medications. The mean BBPS score in the GLP-1 group was 5.2, compared to 6.8 in the non-GLP-1 group (p< 0.05). This suggests that GLP-1 receptor agonists may reduce the effectiveness of standard bowel preparation protocols, potentially due to alterations in gastrointestinal motility [19]. Yao et al., conducted a retrospective cohort study involving 446 patients undergoing colonoscopy. The study compared patients actively using GLP-1 receptor agonists with those who had discontinued these medications at least three months prior. The results indicated a significant difference in BBPS scores, with a mean score of 7.0 ± 1.9 in the GLP-1 group versus 7.5 ± 2.4 in the control group (p < 0.05). Furthermore, 15.5% of the GLP-1 group had a total BBPS score of less than 5, compared to 6.6% in the control group. Additionally, 18.9% of patients in the GLP-1 group required a repeat colonoscopy due to poor bowel preparation, compared to 11.1% in the control group [20]. Byun et al., corroborated these findings, reporting that patients on GLP-1 receptor agonists consistently showed lower BBPS scores, highlighting the need for tailored bowel preparation protocols for these patients [21].
Visualization and Procedural Outcomes
The use of GLP-1 receptor agonists, commonly prescribed for type 2 diabetes management, has implications for bowel preparation and visualization quality during endoscopy. These medications slow gastrointestinal motility, which can lead to an increased presence of residual gastric contents and inadequate bowel preparation, thereby impacting the quality of visualization during endoscopic procedures. A study by Silveira et al., found a significant relationship between perioperative use of semaglutide, a GLP-1 receptor agonist, and the presence of residual gastric content in patients undergoing elective upper endoscopy. The study noted that patients on GLP-1 receptor agonists had a higher incidence of residual food material, which could potentially obscure the visualization of mucosal surfaces and complicate diagnostic assessments [4]. The presence of residual gastric contents not only affects the clarity of endoscopic images but also increases the risk of aspiration during the procedure, as highlighted by Hulst et al. (2021). The study emphasized the need for careful pre-procedural planning and potentially adjusting the timing of GLP-1 receptor agonist administration to mitigate these risks [22].
Sharma et al., investigated the association between GLP-1 receptor agonist use and the incidence of procedural complications during endoscopy. The study involved 400 patients, with 100 patients on GLP-1 receptor agonists. The study found a higher incidence of complications, such as incomplete colonoscopy and need for repeat procedures, in patients on GLP-1 receptor agonists. The increased risk of complications may be related to suboptimal bowel preparation and impaired visualization [23]. These findings highlight the need for heightened awareness and possible protocol adjustments for patients on GLP-1 receptor agonists undergoing endoscopic procedures. Healthcare providers should consider alternative preparation strategies and closely monitor these patients for potential complications. Adjustments in bowel preparation protocols and fasting guidelines may be necessary to ensure optimal visualization and reduce the risk of procedural complications.
Recommendations for Clinicians
Given the impact of GLP-1 receptor agonists on bowel preparation and procedural outcomes, practical recommendations are essential for optimizing endoscopic procedures. Kumar and Lee (2023) provide guidelines for managing patients on GLP-1 receptor agonists, suggesting modifications to bowel preparation protocols. These include longer fasting periods and alternative preparation regimens to enhance bowel cleanliness. Additionally, they recommend withholding GLP-1 receptor agonists prior to procedures to mitigate the risk of delayed gastric emptying and its associated complications [23,24]. Raven et al., further supports these recommendations, emphasizing the importance of considering delayed gastric emptying when managing patient’s peri-operatively. They suggest a 24-hour clear fluid regimen and longer cessation times for GLP-1 receptor agonists to reduce the risk of aspiration and ensure optimal procedural outcomes [25].
Study by Chandrasekhara et al., examining the impact of GLP-1 receptor agonists on endoscopic visualization quality. They found that patients on these medications had a higher incidence of suboptimal bowel preparation, which could lead to incomplete examinations and the need for repeat procedures [26]. The authors recommend considering additional doses of bowel preparation agents and closer monitoring of preparation quality in these patients.
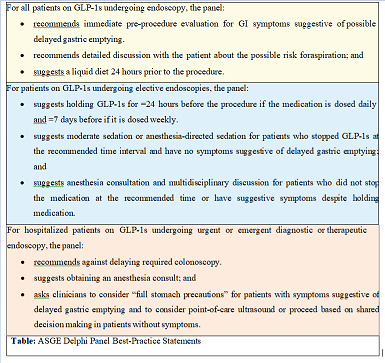
The American Society of Anesthesiologists (ASA) has issued guidance regarding the use of GLP- 1 medications (for type 2 diabetes or weight loss) in patients undergoing endoscopy. Due to concerns about slowed gastric motility and the risk of aspiration from retained gastric contents, the recommendations are as follows: For patients taking daily doses of GLP-1s, consider discontinuing the medication on the day of the procedure while for those on weekly doses, consider stopping GLP-1s a week prior to the procedure. If a patient has not followed these guidelines but shows no gastrointestinal symptoms, proceed with “full stomach precautions” or consider an ultrasound evaluation to assess gastric contents [27].
ArecentclinicalpracticeupdatebytheAmerican Gastroenterological Association (AGA) emphasizes the importance of individualized patient management. This update suggests that while it may not be necessary for all patients to stop GLP-1 receptor agonists prior to endoscopy, careful assessment and potential modification of bowel preparation protocols are recommended to enhance procedural outcomes and reduce. This update came as a result of the American Society For Gastrointestinal Endoscopy (ASGE) convened Delphi panel of 11 experts, from diverse clinical backgrounds to develop best-practice recommendations to clarify the issue for endoscopists.
Conclusion
In conclusion, the current body of evidence highlights the significant impact of GLP-1 receptor agonists on gastrointestinal motility and their implications for endoscopic procedures. While these medications offer substantial benefits in managing type 2 diabetes mellitus, including improved glycemic control and cardiovascular protection, their effect on delaying gastric emptying presents challenges during endoscopy. The increased residual gastric content associated with GLP-1 receptor agonist use can complicate endoscopic visualization, leading to potential procedural complications. This delayed gastric emptying also affects bowel preparation quality, particularly in colonoscopy, where suboptimal visualization may result in incomplete procedures and the need for repeats. As such, clinicians must carefully consider the timing and management of GLP-1 receptor agonists in patients undergoing endoscopy. Adjustments to fasting guidelines and bowel preparation protocols, including extended fasting periods and alternative preparation regimens, are essential to mitigate these risks and enhance the quality of endoscopic outcomes. Overall, this research underscores the need for individualized patient management when using GLP-1 receptor agonists, particularly in the context of endoscopic procedures. Further studies are warranted to refine these protocols and ensure that patients on GLP-1 therapy receive safe and effective care during endoscopic evaluations.
Summary of Findings
GLP-1 receptor agonists have significant effects on gastrointestinal motility and bowel preparation, impacting endoscopic quality. Further research is necessary to refine guidelines and optimize patient management.
Future Directions
Recommendations for future studies to better understand the impact of GLP-1 receptor agonists on endoscopy and to develop tailored bowel preparation protocols for these patients.
References
1. Halawi, H., Khemani, D., Eckert, D., O’Neill, J., Kadouh, H., Grothe, K., ... & Camilleri, M. (2017). Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. The Lancet Gastroenterology & Hepatology, 2(12), 890-899.
2. Seino, Y., Fukushima, M., & Yabe, D. (2010). GIP and GLPâ?1, the two incretin hormones: similarities and differences. Journal of diabetes investigation, 1(1â?2), 8-23.
3. Müller, T. D., Finan, B., Bloom, S. R., D’Alessio, D., Drucker,D. J., Flatt, P. R., ... & Tschöp, M. H. (2019). Glucagon-like peptide 1 (GLP-1). Molecular metabolism, 30, 72-130.
4. Silveira, S. Q., da Silva, L. M., Abib, A. D. C. V., de Moura,D. T. H., de Moura, E. G. H., Santos, L. B., ... & Mizubuti,G. B. (2023). Relationship between perioperative semaglutide use and residual gastric content: a retrospective analysis of patients undergoing elective upper endoscopy. Journal of clinical anesthesia, 87, 111091.
5. Nadkarni, P., Chepurny, O. G., & Holz, G. G. (2014). Regulation of glucose homeostasis by GLP-1. Progress in molecular biology and translational science, 121, 23-65.
6. Alba, M., Ahrén, B., Inzucchi, S. E., Guan, Y., Mallick, M., Xu, L., ... & Goldstein, B. J. (2013). Sitagliptin and pioglitazone provide complementary effects on postprandial glucose and pancreatic islet cell function. Diabetes, Obesity and Metabolism, 15(12), 1101-1110.
7. Camilleri, M. (2014). Gastrointestinal effects of GLP-1- based therapies: Diarrhea, constipation, and gastrointestinal motility. Journal of Clinical Endocrinology & Metabolism, 99(4), 1092-1100.
8. Liu, J., Wang, G., Jia, Y., & Xu, Y. (2015). GLPâ?1 receptor agonists: effects on the progression of nonâ?alcoholic fatty liver disease. Diabetes/Metabolism Research and Reviews, 31(4), 329-335.
9. Goyal, N., Gupta, A., & Mahajan, S. (2016). Novel therapeutic approaches in gastrointes tinal motility disorders: Current and future trends. Current Treatment Options in Gastroenterology, 14(2), 231-243.
10. Hellström, P. M., Hein, J., & Bytzer, P. (2017). Clinical update on GLP-1 receptor agonists in the treatment of gastrointestinal motility disorders. Scandinavian Journal of Gastroenterology, 52(7), 737-747. doi:10.1080/00365521.2017.1301978
11. Nauck, M. A., & Meier, J. J. (2018). Incretin hormones: Their role in health and disease. Diabetes, Obesity and Metabolism, 20, 5-21.
12. Müller, T. D., Finan, B., & Bloom, S. R. (2017). The role of GLP-1 in the regulation of gastric emptying. Diabetologia, 60(5), 825-832.
13. Lee, S. J., Diener, K., Kaufman, S., Krieger, J. P., Pettersen, K. G., Jejelava, N., ... & Langhans, W. (2016). Limiting glucocorticoid secretion increases the anorexigenic property of Exendin-4. Molecular metabolism, 5(7), 552-565.
14. Garza, K., Aminpour, E., Shah, J., Mehta, B., Early, D., Gyawali, C. P., & Kushnir, V. (2022). Glucagon-like peptide-1 receptor agonists increase solid gastric residue rates on upper endoscopy especially in complicated diabetic patients: A case control study. Oficial journal of the American College of Gastroenterology| ACG, 10-14309.
15. Sen, S., Potnuru, P. P., Hernandez, N., Goehl, C., Praestholm, C., Sridhar, S., & Nwokolo, O. O. (2024). Glucagon-like peptide-1 receptor agonist use and residual gastric content before anesthesia. JAMA surgery.
16. Stark, J. E., Cole, J. L., Ghazarian, R. N., & Klass, M. J. (2022). Impact of glucagon-like peptide-1 receptor agonists (GLP- 1RA) on food content during esophagogastroduodenoscopy (EGD). Annals of Pharmacotherapy, 56(8), 922-926.
17. Wu, F., Smith, M. R., Mueller, A. L., Klapman, S. A., Everett, L. L., Houle, T., ... & Hobai, I. A. (2024). Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: a historical cohort study. Canadian Journal of Anesthesia/Journal canadien d’anesthésie, 1-9.
18. Jagtap, N., Kumar, C. S., Kalapala, R., & Reddy, D. N. (2022). Myotomy and EndoFLIP: repeated measurements require a different statistical test. Gastrointestinal Endoscopy, 95(4), 810.
19. Yoshida, S., & Tanaka, S. (2021). Artificial intelligence for the detection of gastric precancerous conditions using image- enhanced endoscopy: What kind of abilities are required for application in real-world clinical practice?. Gastrointestinal Endoscopy, 94(3), 549-550.
20. Yao, R., et al. (2024). GLP-1 receptor agonists and bowel prep: Impact on colonoscopy quality. The American Journal of Gastroenterology.
21. Byun, S. J., et al. (2023). The influence of GLP-1 receptor agonists on bowel preparation quality. Journal of Clinical Gastroenterology.
22. Hulst, A. H., Polderman, J. A., Siegelaar, S. E., van Raalte,D. H., DeVries, J. H., Preckel, B., & Hermanides, J. (2021). Preoperative considerations of new long-acting glucagon- like peptide-1 receptor agonists in diabetes mellitus. British journal of anaesthesia, 126(3), 567-571.
23. Uche-Anya, E., Husby, S., Kaplan, G. G., Underwood, F. E., Green, P. H., & Lebwohl, B. (2021). An international reporting registry of patients with celiac disease and COVID-19: initial results from SECURE-CELIAC. Clinical Gastroenterology and Hepatology, 19(11), 2435-2437.
24. Kruger, A. J., Abougergi, M. S., Jalil, S., Sobotka, L. A.,Wellner, M. R., Porter, K. M., ... & Mumtaz, K. (2023). Outcomes of nonvariceal upper gastrointestinal bleeding in patients with cirrhosis: a national analysis. Journal of Clinical Gastroenterology, 57(8), 848-853.
25. Raven, L. M., Brown, C., & Greenfield, J. R. (2024). Considerations of delayed gastric emptying with periâ? operative use of glucagonâ?like peptideâ?1 receptor agonists. Medical Journal of Australia, 220(1), 14-16.
26. Chandrasekhara, V., et al. (2022). Impact of GLP-1 receptor agonists on bowel preparation quality for endoscopy. Gastrointestinal Endoscopy.
27. Ushakumari, D. S., & Sladen, R. N. (2024). ASA consensus- based guidance on preoperative management of patients on glucagon-like peptide-1 receptor agonists. Anesthesiology, 140(2), 346-348.



