Research Article - (2024) Volume 9, Issue 2
Survival and Concomitant Antitumor Immunity in Cancer
Received Date: Nov 22, 2024 / Accepted Date: Dec 13, 2024 / Published Date: Dec 20, 2024
Copyright: ©©2024 Kangla Tsung, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Tsung, K., Xu, Z., Hui, Z. (2024). Survival and Concomitant Antitumor Immunity in Cancer. J Clin Exp Immunol, 9(2), 01-19.
Abstract
What determines the survival time in a cancer case? An obvious aspect is the malignancy of the tumor. Based on our clinical observation, this so-called malignancy includes three aspects: 1) The mode of tumor replication, i.e., how rapidly a tumor replicates; 2) The ability to form distant metastasis; and 3) The ability to cause symptoms, often related to the ability to drive local inflammation. But tumor malignancy alone does not seem to correlate with cancer survival. Another factor contributing equally critical or even more critical to cancer survival is often ignored. It is the status of host antitumor immunity. Tumor malignancy is only the determinant factor for cancer survival when concomitant antitumor immunity is absent in a case. With a strong antitumor immunity, regardless of tumor malignancy, a case is likely to survive much longer than that of a case with a week or none antitumor immunity. Thus, antitumor immunity is a more influential factor than tumor malignancy on cancer survival. Yet, the status of antitumor immunity has been consistently ignored in clinical management of cancer and still is as of today. Unless it is clearly demonstrated in cancer cases that the lack of antitumor immunity is the most critical factor influencing survival, clinicians are unlikely to pay attention to the status of antitumor immunity in each case, less to say to manage accordingly. There have been many previous studies to link immunity to cancer survival in numerous statistical analyses, but these findings did not raise alert in clinicians to the point that the status of antitumor immunity is being considered in selection of treatment plan when it comes to individual cases. In this report we try to describe few individual cases in which the status of antitumor immunity is clearly responsible for survival. We hope the conmen aspects and clear contrasts in these cases will bring a more vivid picture to clinicians who will then realize how important is the status of antitumor immunity in each cancer cases and learn to select treatments based on the status of that immunity.
Keywords
Cancer Survival, Concomitant Antitumor Immunity, Autonomous Tumor Replication, Cancer Metastasis, Immune Checkpoint Pathway, Cancer Recurrence
Introduction
Clinical observations show that the real-world survival times for individual cancer cases vary widely even among patients with similar TNM staging designations. The actual survival time for each individual case is so unpredictable that it is rarely discussed or even mentioned in the medical literature. Except for a few really desperate late-stage cases, no doctors in today’s clinic can answer with certainty the question of “how long to I have” from most newly diagnosed patients, simply because he does not know. Some seemingly desperate cases may turn out to last way longer than expected while some other cases without even symptoms may have a sharp turn and quick death.
What factor(s) contribute to cancer survival remains a mystery till now. On the other hand, it is a common belief that the degree of malignancy of a tumor should contribute to its growth and thus influence patient’s survival. It is generally true that a fast growing and rapidly metastasizing tumor leads to rapid deterioration of its host and death. But out of such extreme cases, the actual survival times for many seemingly “normal” cases are widely variable that one can hardly put on a number unless it is approaching the terminal stage with severe symptoms and cachexia. Due to such uncertainty, the medical society continue to ignore individual survival but to compile a group survival for patients with similar characteristics such as same TNM staging. The most popular Kaplan-Meier survival curve used in millions of medical studies best reflect this approach. Are individual survival times truly unpredictable? Observations based on our own research during the past decade have pointed to the status of host antitumor immunity as the most influential factor on patient survival. While individual status of antitumor immunity is variable because of genetic variation, it can be detected and evaluated individually. Thus, by theory, the survival time for individual cancer case based on individual status of antitumor immunity should be predictable.
In many grouped retrospective analyses of cancer recurrence following surgery, the presence and degree of immune infiltration clearly stand out to be decisive factor preventing recurrence [1-7]. Since long disease-free survival is directly linked to long overall survival, one can say with certainty that the status of antitumor immunity at the time of surgery should be directly responsible for post-surgery survival. This conclusion has been confirmed in animal tumor model by us previously too [8]. But until now, no individual survival time has been linked to the status of antitumor immunity and predicted for individual cases. This is due to two reasons: one is that there are hardly adequate and accurate measurements for status of antitumor immunity, thus clinicians do not have a way to gauge the levels of antitumor immunity individually. The second reason is that the importance of this measurement is not recognized by the medical society. Thus, the situation becomes deadly locked in that if they don’t see it, they do not realize its importance, so they don’t want to see it. For tumor immunologists, the antitumor immunity is clearly important because they see it and they analyze its roles in tumor models for decade [9-15]. One of the most obvious way to find out the importance of antitumor immunity is to delete it in animal tumor models to see what happens. By doing this, they know that antitumor immunity is critical for tumor control such as prevention of the growth of primary tumor andthe establishment of metastasis [8,13].
But despite thousands of studies in animal models, this knowledge has not translated into clinical management of cancer. Other than the lack of accurate assessment/measurement of antitumor immunity in a clinical setting, the persistent belief that spontaneous human tumors are not immunogenic, thus antitumor immunity plays almost no roles in cancer management has also occupied the field for a long time [16-18] until the demonstration of the power of antitumor immunity in large number of late-stage cancer patients by the immune checkpoint blocking (ICB) antibody therapy in recent years. Even though, the general belief is still that antitumor immunity plays almost no role in tumor control until it is activated by so-called immunotherapy such as tumor vaccine or ICB antibody.
Like in animal tumor models, the demonstration of any possible roles by concomitant antitumor immunity in its natural (i.e., during persistent tumor-bearing) state or during cancer therapy (for example during traditional tumor reductive therapies) relies on a direct comparison before and after this immunity is removed. In animal models, this is done by removing T cells through various means such as thymectomy, sublethal radiation and antibodyinduced cell clearance via antibody-dependent macrophagemediated phagocytosis (ADCP) and antibody-dependent cell cytotoxicity (ADCC). For obvious reason, we cannot deliberately delete antitumor immunity in a patient by these means in order to find out the consequence.
Thus, we have to turn to group comparison between patients with and without antitumor immunity to see which group has better prognosis. This comparison is very rough since antitumor immunity is genetically different among individual patient by theory. Yet, such comparison still provided a general assessment of the contribution of concomitant antitumor immunity towards prognosis. Many studies have shown that patients with large number of tumor-infiltrating T cells in the resected tumor sample will likely have much delayed post-surgery recurrence in multiple solid tumors compared to patients who do not have such T cell infiltrates in their tumor [1-7]. Despite these findings, the significance of antitumor immunity in the natural tumor-bearing state in a given patient remains unknown.
The development of ICB antibody therapy in recent years has created a situation to view this direct comparison un-intentionally. Due to the true mechanism unknown to the mainstream medicine, a situation of immunity depletion has been created in many cases [19]. This is the true reason for the so-called hyper-progression associated with ICB antibodies [20]. This situation closely mimics the T cell depletion tumor immunologists create in animal models. For those clinicians who have wrongly applied the ICB therapy to cause hyper-progression and quick death of patients, the horrifying consequence should be very impressive. Had they known that it is the consequence of a loss of antitumor immunity, there is nolonger need to prove to them the critical role of antitumor immunity in human cancer. Once a malignant tumor is defined, the presence or a strong antitumor immunity and the absence of antitumor immunity create the two extremes for cancer prognosis. By saying so, we acknowledge two major factors that contribute to cancer survival: tumor malignancy and status of antitumor immunity. The malignancy of a tumor is a necessary factor for poor prognosis since if a tumor is benign, the status of immunity is not critical. On the other hand, the status of antitumor immunity is sufficient to determine the outcome of a tumor, as long as it is malignant.
Here we illustrate this relationship between these two factors using individual cases. In this report, we choose five ovarian cancer cases to show three points: 1) When the tumor is not highly malignant, meaning the ability to establish distant metastasis is missing, patient survival could be long even without the help of antitumor immunity. 2) When the tumor is malignant, presence of antitumor immunity is critical for patient survival. 3) Just having a strong antitumor immunity may not guarantee a good prognosis since residual tumor cells following removal of primary tumor lesion by surgery often express immune checkpoint molecule (PDL1) when attacked by strong immunity and present as persistent “recurrence”. Knowing this possibility and dealing with proper treatments are necessary to obtain a good prognosis.
Case Description
Case 1: Long-Term Survival when Tumor is not Malignant
A 40+ year old woman sought medical treatment for abdominal pain and ascites and was suspected for ovarian malignancy. The primary ovarian lesion and the visible pelvic metastases were surgically removed. Postoperative pathology showed a moderately and poorly differentiated high-grade serous carcinoma, FICO stage IIIc. After the operation, eight consecutive chemotherapy sessions were performed according to the guidelines, which then changed to intermittent chemotherapy once every 2 to 3 months for 12 months. Multiple pelvic metastases plus distant dorsal metastases were noted 3 months after the last chemotherapy. As a result, a second operation was performed, and chemotherapy was given nine times after the operation. Four months after chemotherapy was stopped, recurrence and metastasis occurred again in the spleen, liver, and pelvis. A third surgery was performed followed by seven more rounds of chemotherapy. Examination one month after the last chemotherapy revealed recurrences in the liver, pelvic and rectal metastases plus a possible brain metastasis. After radiation treatment of brain metastasis, and the fourth operation of the abdomen and pelvis, six rounds of chemotherapy were given. The patient began to take targeted drugs thereafter. A fifth operation was performed a year later to resolve intestinal adhesions, and metastatic nodules were found during the operation. A combination of chemotherapy and targeted drugs were prescribed. The patient is still alive with apparently recurrence-free status after more than 7 years to date.
Why can an "advanced" ovarian cancer survive ongoing recurrences for more than 7 years? It can't be the treatment that is highly effective. If one could have survived for 7 years with multiple surgeries and chemotherapy, other cases would have done so, too. It is not the timely identification of recurrence that one can live over 7 years. If so, other cases with sensitive tumor markers would have done the same. Chemotherapy was also performed using first-line regimens and the targeted agents were almost ineffective (relapsed during use). In addition, it was not the immune control, because there was no reasonable concomitant immunity seen from the beginning of this case. The fundamental reason for the longterm survival with tumor in this case was that the primary tumor was not highly malignant. Let's look at the structure, the mode of tumor replication and status of antitumor immunity of the primary tumor.
This is a typical well-differentiated serous adenocarcinoma (Figure. 1-1, HE) with no positive signal for Ki-67 staining (Figure. 1-1, Ki-67), which indicates no autonomous replication, but with a strong positive PCNA staining (Figure. 1-1, PCNA), representing a highly active non-autonomous replication [21,22]. At the same time, there were almost no T cells in the tumor area (Figure 1-1, CD3).
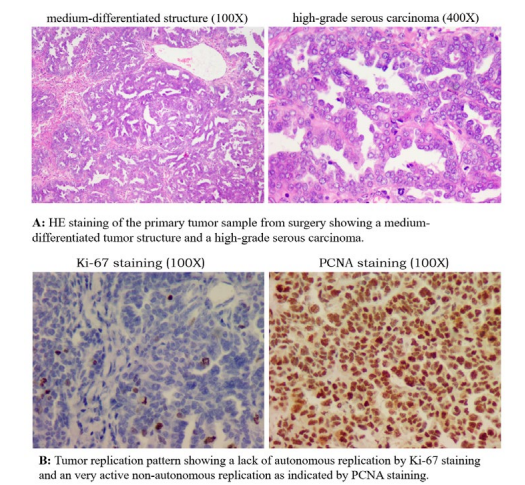
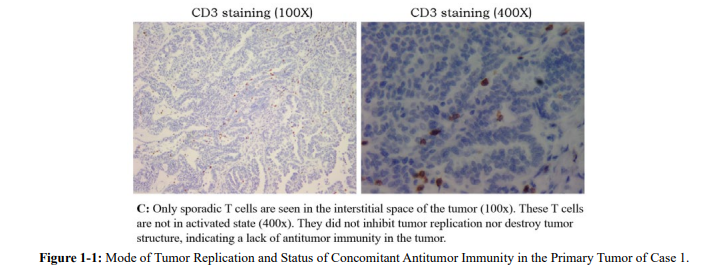
These observations pointed to an active local tumor with deficient autonomous replication. Tumor growth relied on non-autonomous replication driven most likely by local inflammation of the ovary (an even often found in the history of many ovarian cancer cases). There was neither immune recognition nor distant metastasis. Years of observation by TANLUN have shown that autonomous tumor replication is absent in approximately one third of ovarian cancer cases. These cases have either no distant metastasis, or the distant metastasis (often single) has autonomous replication without exception [22].This is also true in this case when we looked at the staining of the distant dorsal metastasis from the second operation. Tumor structure was similar to that of the primary lesion with more interstitial space (Figure. 1-2, HE). At least 20% of Ki-67- positive cells are present in tumor tissue (Figure. 1-2, Ki-67), and non-autonomous tumor replication (PCNA staining) remains highly active (Figure. 1-2, PCNA).
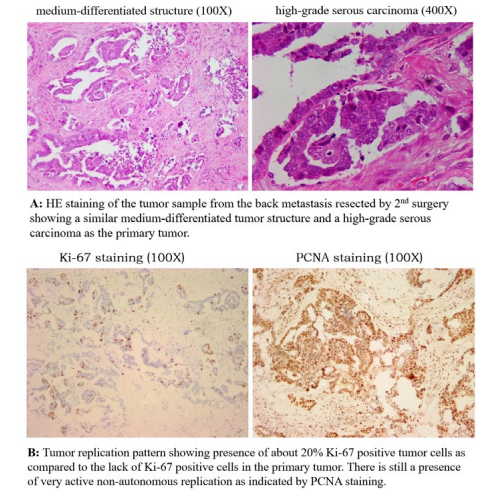
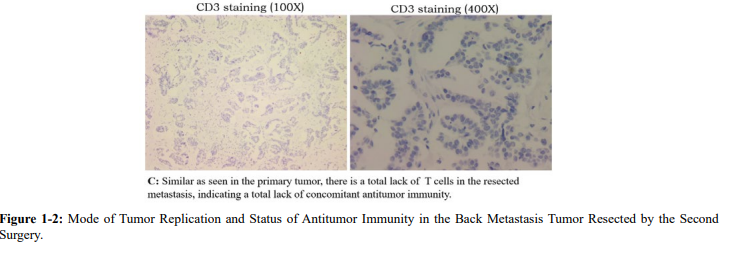
The low number of T cells suggests that concomitant immunity was still absent. The question then is how did the autonomous replication of the metastasis come about? It is unlikely from the primary lesion because in that case we should see multiple metastases instead of one. The autonomous replication was most likely derived from non-autonomous replication with mutation that was caused by chemotherapy. We have previously reported a similar case [22]. The presence of autonomous replication in the distant metastasis suggested that it was a component "distinct" from the main components of the primary lesion. If the source of this autonomous replication is from primary lesion, there would not be a single metastasis, but other multiple metastases. On the other hand, these autonomous replication variants would not be frequent if they had come from chemotherapy-induced genetic mutations.
In addition, the metastatic lesions in the second operation still lacked strong immune recognition, so postoperative protection could not be provided to avoid other recurrence had they existed. After the second surgery, nine rounds of chemotherapy were again performed, which made it possible to induce more mutations leading to autonomous replication. Four months after chemotherapy was stopped, recurrence in the spleen and pelvis were detected again, followed by a third operation in which metastases from the spleen and pelvic colon wall were removed. Pathology showed active autonomous replication of the tumor without concomitant immunity.
Chemotherapy was given again after the operation.Recurrence in the pelvis was noted again several months after the chemotherapy and a fourth operation was performed. Post-surgery pathology only revealed sporadic “suspicious” metastases. For this reason, after the fourth surgery, chemotherapy was switched to targeted therapy with PARP inhibitor. From the history of post-surgery management, every postoperative chemotherapy will have the opportunity to produce mutations that generate autonomous replication.On the other hand, generation of mutation by chemotherapy requires active tumor replication. Since all naturally active replicating tumor cells or tumor cells stimulated by chemotherapy-induced inflammation were depleted with time,the frequency of new occurrence will be lower and lower with time.
About a year after the fourth surgery, bowel obstruction occurred, and the hospital suspected another recurrence and did a surgical exploration to alleviate the obstruction. The obstruction was caused by adhesion, not massive tumor recurrence, but several suspected nodules were identified in the pelvis and were excised during the operation. Pathology revealed the presence of moderately differentiated adenocarcinoma in the nodules (Figure. 1-3, HE). These so-called metastases have no sign of either autonomous or even non-autonomous replication (Figure. 1-3, Ki-67 and PCNA).
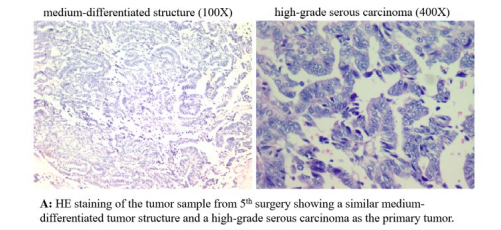
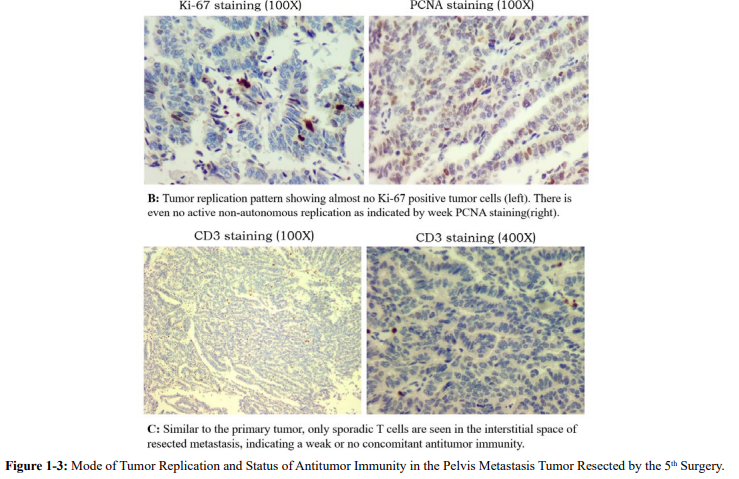
This indicates that the previously non-autonomously replicated nodules left behind by the previous primary lesion can exist for a long time, and it cannot be ruled out that there will also be the formation of individual slow-growing autonomous replicated lesions that were induced by chemotherapy. Even so, there is a high probability that this will only be a single nodule. As long as there is no longer an autonomous replication mutation, there will be no real "relapse". And in order to avoid the occurrence of mutations leading to autonomous replication, chemotherapy should not be prescribed.
A case of stage IIIc high-grade serous carcinoma of the ovary, with no concomitant antitumor immunity involved in protection and control from the beginning, has survived for more than 7 years since its diagnosis and is still living without sign of tumor. The long-term survival is likely due to the lack of autonomous replication of the primary tumor, i.e., tumor is not highly malignant according to our estimate. The first three recurrences after surgery were likely due to individual mutations induced by postoperative chemotherapy. Had post-surgery chemotherapy not performed in this case, there could be no recurrence to begin with. The last two “recurrences” were not true relapses driven by autonomous replication. Such multiple recurrences did not result in an outbreak of uncontrolled metastases and death. Similar cases of tumors not replicating autonomously, and had survived for a long time have been observed and reported by us before [22]. This is the reason we emphasize that antitumor immunity is only predictive for prognosis when malignant tumor is present. Under such a prerequisite, presence or absence of concomitant antitumor immunity becomes critical for patient survival. The following cases will demonstrate this point.
Case 2: Short Survival in Malignant Tumor
when Antitumor Immunity is Absent A young woman of less than 30-years old went to the clinic for persistent abdominal pain. An ovarian mass was found by imaging and removed by surgery. The postoperative pathology revealed a high-grade serous carcinoma with multiple pelvic metastases, and a FICO stage III was assigned. Based on the guideline, the hospital prescribed six courses of platinum-based first-line chemotherapy after the operation, but sensitive tumor marker CA125 failed to decrease to single digit after chemotherapy.
Tumor marker began to rapidly increase a couple of months later, and chemotherapy was started again for another four courses. Tumor marker continuously increased during chemotherapy, demonstrating a platinum-resistant recurrence. The patient went to us for further treatment advice. First, we checked the mode of tumor replication and the status of concomitant antitumor immunity in the removed primary tumor. This is a tumor of high-grade serous carcinoma (Figure. 2, HE) with highly active autonomous replication (Figure. 2, Ki-67). Because there are no T cells present in the tumor area (CD3 staining), this case lacks antitumor immunity. This combination of high malignancy and lack of antitumor immunity made postoperative recurrence inevitable.
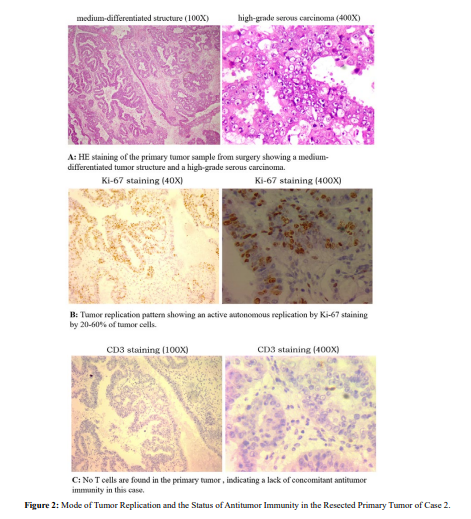
Chemotherapy itself can slow down and prevent the establishment of new metastases in a short period of time, but once chemotherapy stops, metastases will start to rebound and establish again. Once the lesion is established, chemotherapy loses control through direct killing. Chemotherapy efficacy depends on the activation of pre-existing anti-tumor immunity [23,24]. In this case, antitumor immunity cannot be activated because there is no concomitant immunity to begin with. Generally, this lack of tumor response to platinum-containing chemotherapy in recurrent ovarian cancer is found to have a bad prognosis with survival period of only 8 to 10 months since recurrence. Lack of antitumor immunity is likely the true reason behind.
With these observations, especially the lack of antitumor immunity in this case, we recommended that the patient switch to intermittent chemotherapy, waiting for immune recognition to establish, if it could. Immune recognition can be reflected by the spontaneous decline of tumor marker. After eight months of intermittent chemotherapy, immune recognition was still absent with progressive tumor burden development, but the patient's quality of life was maintained. Thereafter ascites and liver metastases developed and the patient died 2 months later. The case survived for 14 months after recurrence. By contrast, the following three ovarian cancer patients with concomitant immunity were lucky.
Case 3: Weak Antitumor Immunity Combined with Intermittent Chemotherapy Provided Long Term Post-Surgery Protection Against Recurrence
A 50+-year-old woman with persistent abdominal pain and constipation went to the hospital. A large cystic lesion of >10 cm in the pelvis and multiple solid nodules were noted. Tumor marker CA125 (> 1000) was significantly elevated. Surgical exploration confirmed that most of the nodules were malignant tumors, and the largest lesion was located in the intestinal wall. Postoperative pathology showed that the tumor was poorly differentiated highgrade serous carcinoma, originating from the ovary, and multiple pelvic metastases were identified. After surgery, the family members went to us for advice on postoperative treatment options.
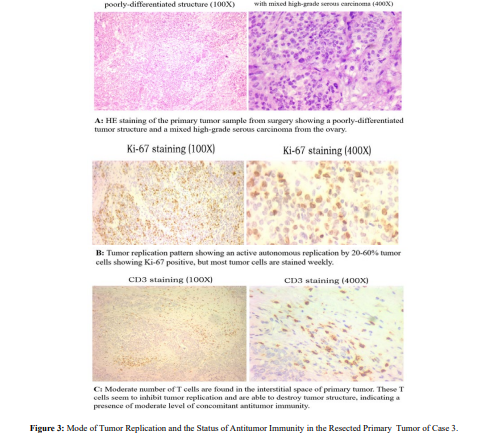
We first looked at the mode of tumor replication and status of concomitant immunity from the surgical sample. As Figure 3 shows, tumor morphology shows a high-grade serous carcinoma (HE). Tumor replication is highly active (Ki-67), consistent with presence of multiple metastases in the pelvis. Different from the above tow cases, this case had concomitant antitumor immunity present in the tumor, albeit the number of T cells is not massive (CD3), but they seem to have ability to inhibit tumor replication (note that Ki-67 positive tumor cells near T cell area seem to have less intensive Ki-67 staining, a sign of lower rate of replication).Based on our experiences, such level of concomitant immunity could provide postoperative protection for recurrence in the short to medium term (within 2 years), but not for long term (>3 years). Postoperative chemotherapy can help remove some latent metastases in advance and delay recurrence. However, long-term protection requires 2-4 years of intermittent chemotherapy following the two-year immune protection period. Our patient followed our recommendation with three postoperative chemotherapies followed by tracking tumor marker (CA125) change for two years. After this period,intermittent chemotherapy every 2 to 3 months each round was started. It was intended for a period of at least 3 years but was interrupted due to the Covid-19 epidemic after two years. Tumor marker rebounded 4 months later after the last chemotherapy, indicating a recurrence had taken place due to interruption of intermittent chemotherapy. Targeted therapy and occasional chemotherapy were combined to continue management of recurrence, and the tumor marker fluctuated slightly exceeding the normal range. Tumor marker rapidly elevated 7 years after surgery and imaging examination revealed a single recurrent lesion at the surgical site. The patient is currently under treatment for this recurrence.
As in the previous case, this case was also a high-grade serous carcinoma with similar malignancy based on mode of tumor replication and presence of multiple pelvis metastases. It is because that there's an antitumor immunity that this case can survive for more than seven years and more. The level of antitumor immunity at the time of surgery was not strong but enough to provide at least two years of post-surgery protection against recurrence. We knew that this immunity was not strong enough to provide long-term protection and therefore recommended intermittent chemotherapy to take over after two years following surgery. This treatment apparently was effective because recurrence took place after this intermittent chemotherapy was interrupted by Covid-19 epidemic. Had this treatment not been interrupted, recurrence of this case may not occur or at least delayed for the period of intermittent coverage (another 2 years). By then, it was more than 5 years after removal of primary tumor, disseminated tumor deposits may be depleted and a clinical cure may be obtained [8]. Because that this case has a weak antitumor immunity at the time of surgery, one may ask: if there is a strong antitumor immunity, will that secure a clinical cure after surgery? Although theoretically speaking this is true, but in reality, this is often not the case. It is true only when complete surgery (R0 resection) is achieved. Yet in reality, due to large tumor burden, severe ascites and local spread in most ovarian cancer cases, tumor resection is often incomplete. With a strong antitumor immunity remaining, the residual tumor lesions always express immune check point molecules such as PDL1, which leads to resistant to elimination by immunity.
The progression of residual tumor creates impression of tumor recurrence, often much earlier than the true recurrence of newly established metastasis following decay of antitumor immunity. However, even with postoperative recurrence, the overall prognosis for these cases of strong antitumor immunity is better than those without antitumor immunity. If the management is wrong, the recurrence may be out of control. In contrast, if the management is correct, clinical cure is possible. The following two cases illustrate postoperative recurrence and different responses with strong antitumor immunity.
Case 4: High Malignant Tumor with Strong Antitumor Immunity Recurred Due to Residual Tumor
A 50+-year-old woman with abdominal pain, abdominal distension and virginal bleeding went to the hospital., A lesion over 10 cm in one ovary was identified. Surgical exploration was arranged and standard radical surgery for ovarian cancer was performed after the malignancy was confirmed during the operation. Postoperative pathology revealed a high-grade serous carcinoma with extensive pelvic metastasis, leading to FIGO stage IIIc assignment. The hospital prescribed standard postoperative chemotherapy. But after the 4th round, the patient experienced intolerance to further chemotherapy, and her family members came to us for consultation.
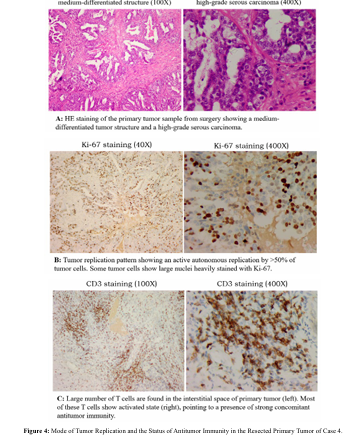
We examined the mode of tumor replication and the status of antitumor immunity in the resected primary lesion. The tumor presented with high-grade serous carcinoma (Figure. 4-1, HE) and highly active autonomous replication (Figure. 4-1, Ki-67) and a large number of T cells (Figure. 4-1, CD3) in the tumor area. These observations pointed to a highly malignant tumor with active replication, but strong concomitant antitumor immunity at the time of surgery. Despite the massive presence of T cells in the tumor, the Ki-67 staining color of many tumor cells was markedly increased with the presence of T cells nearby, indicating these T cells seem to stimulate instead of to suppress tumor replication.
In addition, this case also presented with multiple pelvis metastases, indicating that tumor spread had taken places before establishment of immune recognition. Based on the presence of a strong antitumor immunity,more rounds of chemotherapy are not necessary to prevent recurrence, because this antitumor immunity has the ability to inhibit new metastasis for a protective period of at least 4 years based on our estimates. However, if surgery is incomplete and there is residual tumor, the tumor cell will likely express PD-L1 due to strong immune attack-associated local release of IFN-gamma [25].
This tumor expression of immune check point molecule is the main reason for the recurrence in many cases despite the strong antitumor immunity following surgery [26]. In this case, there was extensive pelvic metastasis before surgery, and it was difficult to operate without leaving any residual tumor. Postoperative chemotherapy is only effective preventing establishment of new metastasis, but Figure 4-2: Why can't concomitant immunity eliminate residual lesions while able to destroy new metastases? cannot eliminate existing residual tumor. Our patient discontinued chemotherapy as recommended, and tumor marker was followed with regular follow-up imaging. Tumor marker increased slowly after one year. This recurrence should not be a recurrence due to immunity decay but rather a slow progression due to the expression of PD-L1 by residual tumor. The correct management should have been active elimination of the residual tumor to secure a clinical cure, but the patient herself was pessimistic and gave up further exam and treatment, leading to her eventual death two years later.
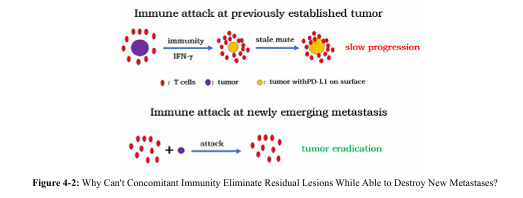
This case was also a high-grade serous carcinoma of the same malignancy from the view point of tumor replication as the previous two cases. However, despite the stronger concomitant immunity at the time of diagnosis and surgery, this case also had recurrence, which should be due to the PD-L1 expression caused by immune attack on the residual lesion. Why can't concomitant immunity eliminate residual lesions while able to destroy new metastases? It is a matter of timing for immune recognition and lesion establishment.
As Figure 4-2 illustrates, if the lesion is established before immune recognition and attack, this lesion cannot be eliminated in the f irst place. The established tumor cells are stimulated by gamma interferon from immune attack to express PD-L1, protecting tumor cells being efficiently inhibited or even killed by immunity [26]. As consequence, the established lesion is likely form stalemate with antitumor immunity as we have shown before [19]. On the other hand, if immunity is established already, new metastases will not express PD-L1 until they are attacked by the existing immunity. Once it is recognized by immunity, it is rapidly eliminated due to low tumor burden (direct killing + replication inhibition). PD-L1 has no chance to be expressed before tumor elimination. This case belongs to the former situation while Case 3 belongs to the latter situation.
There are many similar cases of recurrent ovarian cancer, and few have a direct clinical cure unless there is no widespread pelvic metastasis (for example, FOGO stage I or II). Not only the patients, but also the clinicians take the cases of recurrent ovarian cancer with a desperate attitude. However, as long as that the recurrence is caused by the expression of PD-L1 in a single residual lesion, this recurrence can be actively managed, and a clinical cure is possible, as is illustrated in the following case.
Case 5: Correct Management for Strong Antitumor Immunity-Induced Recurrence
A 50+-year-old woman sought for medical treatment due to abdominal pain. An ovarian mass and ascites were discovered. Tumor marker CA125 was over 1000. The hospital prescribed two rounds of chemotherapy. The ascites disappeared and CA125 decreased significantly. The hospital then arranged surgical resection.
Postoperative pathology showed a poorly differentiated ovarian fallopian tube carcinoma without extensive pelvic metastasis. The case was designated as stage II. CA125 further decreased after surgery, but still exceeded the normal range.
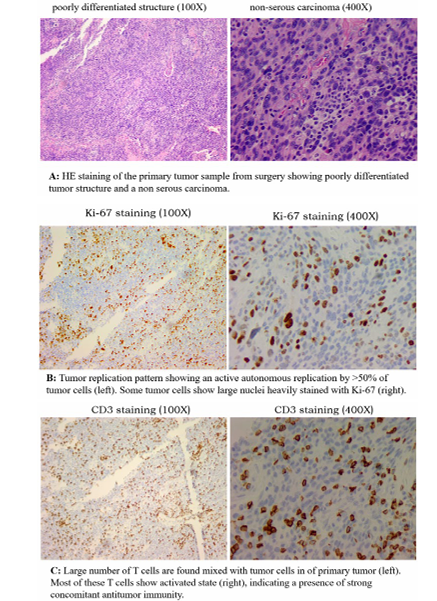
Figure 5-1: Mode of Tumor Replication and the Status of Antitumor Immunity in the Resected Primary Tumor of Case 5
Chemotherapy was then prescribed. CA125 did not decrease but increased during chemotherapy, and family members came to us for further treatment suggestions.We first evaluated the mode of tumor replication and the status of antitumor immunity in the resected primary lesion.It was a poorly differentiated tumor (Figure. 5-1, HE) with active tumor replication (Figure. 5-1, Ki-67), possibly a highly malignant case. However, there was also a strong presence of antitumor immunity. A large number of T cells (most of them were CD8 subtypes) was seen, with some showing activated states (Figure. 5-1, CD3).
There was no sign of T-cell-mediated destruction of the tumor (scar fibrosis and interstitial space formation) and no evidence that tumor replication was inhibited by the presence of T cells, but rather stimulation of tumor replication (a sign of PD-L1 expression by tumor cells).The absence of extensive pelvic and distant metastasis is likely due to the action of this antitumor immunity. Ascites and abdominal pain prior to the diagnosis were associated with increased inflammation, which was caused by increased replication of the established lesions.
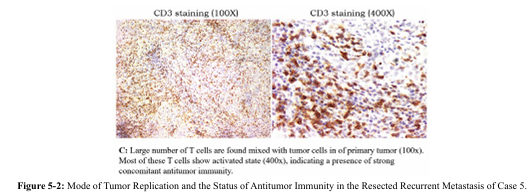
Increased inflammation without concomitant extensive metastasis indicated the presence of immune control. The most plausible explanation is that strong antitumor immunity attacks the tumor, resulting in increased tumor replication after PD-L1 expression, leading to increased release of inflammatory chemotic factors Figure 5-2: Mode of tumor replication and the status of antitumor immunity in the resected recurrent metastasis of Case 5. by tumor cells, and increased local symptoms.The failure of postoperative CA125 to decrease to a low level (single digit) indicated the presence of residual lesion(s). The tumor marker rebounded from the postoperative chemotherapy because that chemotherapy further activated immunity, which in turn increased the attack on the residual tumor,
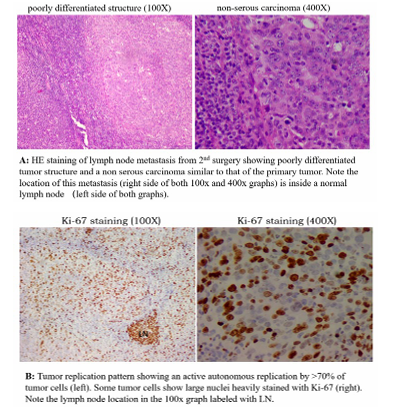
An increase in PD-L1 leads to an increase in tumor replication, which is reflected in an increase in tumor mark. Based on this judgement, we predict the that a single residual lesion will be detected over time. Chemotherapy could not inactivate the existing residual lesions because it would activate the immunity and induce the tumor to express PD-L1. Therefore, we suggest performing intermittent chemotherapy till we can find the residual lesion. Tumor marker increased slowly during intermittent chemotherapy about one year before stabilized. A PET-CT scan revealed an isolated pelvic metastasis with active metabolic signal.
This single lesion was subsequently excised by surgery. Post operation pathology analysis confirmed a tumor-immune standoff with both active tumor replication and large number of activated T cells infiltrating the tumor (Figure. 5-2). The postoperative tumor marker was reduced to a low level below normal range and no other adjuvant treatment was given. Patient remained recurrence free for 45 months since the second operation, and the overall survival period since the initial diagnosis is more than 7 years by now.
This was a case of a highly malignant tumor but with strong antitumor immunity. Without a stronger concomitant immunity, distribution of metastases at the time of diagnosis would not have been limited. Also, without a strong antitumor immunity, extensive recurrence and metastasis would have occurred shortly after surgery to remove primary tumor. Also, because that the attack by the stronger antitumor immunity caused the residual tumor to express PD-L1, any residual tumor may not be inactivated by residual antitumor immunity. As long as the residual lesion is removed, a tumor-free state is reached. The remaining strong antitumor immunity could maintain this state (Figure 4-2), leading to a clinical cure after the secondary surgery.
Discussion
We intend to use the above five cases to demonstrate the critical role of antitumor immunity in patient survival. We believe that this relationship could be demonstrated by the “traditional” group comparison. The reason such comparison has not been presented is that status of antitumor immunity in the individual case is not recognized as a critical parameter and the method to measure it has not been established. Had such measurement established, one can easily separate cases into two groups according to the levels of concomitant immunity. Another issue that complicated this comparison is the confusion brought by tumor expression of PD L1, which often result in earlier recurrence and resistance to various treatments due to resistance to immune attack [27]. Activated immunity is the true reason for tumor responses to traditional tumor-reductive therapy [24]. Resistance to immune attack by immune checkpoint pathway is the true reason behind lack of continued tumor response to most tumor reductive therapies. If a tumor has the ability to drive inflammation through releasing chemotactic factors during active replication, the consequence of tumor expression of PD-L1 would be increased local inflammation that gives the impression of disease progression. Mis-management of such situation often leads to shortened survival. When strong antitumor immunity is associated with short survival in some cases, the claim that patient survival is directly related to the levels of antitumor immunity becomes false and confusing [27]. Only when the situation of immunity-induced tumor expression of PD-L1 is recognized and separated from those cases in which immunity does not induce tumor expression of PD-L1, one can again see the true relationship between levels of antitumor immunity and patient survival. Even with tumor expression of PD L1, proper management may still produce good prognosis as Case 5 demonstrates. It should be noted that contrary to general belief, immune checkpoint blocking (ICB) therapy with antibodies is not a guaranteed treatment to counter PD-L1-memdiated immune resistance as this therapy may cause over depletion of antitumor T cells leading to loss of tumor control [19].
The best way to resolve the resistance by PD-L1-expressing tumor is to eliminate the tumor by surgery or radiation therapy. As such, the degree of resection (for example R0 resection) becomes critical. Complete surgery without visible residual tumor is preferred but may not be possible in many cases such as in extensive ovarian cancer like in the cases cited here. Similar situation is often found in breast cancer surgery due to micro metastases spread under skin. With the introduction of post-surgery radiation, the cure rate of breast cancer has significantly improved. The true reason is likely the destruction of PD-L1-expressing residual tumor.
Ironically speaking, the critical role of antitumor immunity in cancer control has been recognized for over 70 years in animal studies [10], and there is an independent research area called “Tumor Immunology” well recognized by the research and medical fields with hundreds and thousands of publications each year. Yet, there is not a single case management in cancer clinic today that incorporates the status of antitumor immunity during the course of patient management. Even the selection of the so called immunotherapy does not consider the true tatus of antitumor immunity of a patient, less to speak about the selection of traditional tumor reductive therapies. Is the status of antitumor immunity in each cancer patient not critical for their survival? Or it is only important in research animal models? The lack of established measurement for levels of antitumor immunity in cancer patient is a factor, but it is not the preventive reason. The real reason is the general belief that the role of antitumor immunity in the natural tumor-bearing state of a cancer patient is trivial at best if not negative. Prominent tumor immunologist has repeatedly argued that based on observations from animal tumor models, antitumor immunity is relevant only in chemically induced tumors and naturally developed tumors often do not have concomitant antitumor immunity during its growth in syngeneic hosts that inhibits tumor growth [11,16]. Instead, attempt to immunize hosts against these tumors may actually stimulate tumor growth [17,18]. Since human cancers are mostly naturally developed, antitumor immunity should not play any significant role during tumor growth. Even deliberate tumor vaccine therapy may not have important impact. This pessimistic view, combined with repeated failure in various tumor vaccination strategies, has become the public opinion in cancer clinical field for mostly naturally developed, antitumor immunity should not play any significant role during tumor growth. Even deliberate tumor vaccine therapy may not have important impact. This pessimistic view, combined with repeated failure in various tumor vaccination strategies, has become the public opinion in cancer clinical field for many years till the recent explosive adaptation of ICB therapy in cancer management demonstrated how powerful antitumor immunity may be if activated properly. These exciting observations have completely overthrown the previous pessimistic view on the role of antitumor immunity in cancer management. Today, there is no need to “persuade” any cancer clinician on how important antitumor immunity is. But, still there is not a single case in which the status of antitumor immunity of the patient is considered during therapy selection process. Besides the fact that there is still lack of established evaluation on the status of antitumor immunity in individual patient, there is also a conceptual gap in the possible role of antitumor immunity in the natural tumor-bearing state of a patient. Most people take the miracle effect of ICB therapy as the consequence of immunity activation. They often ignore the role the antitumor immunity has before it is activated. They don’t even know whether antitumor immunity is concomitant or it is created by therapy.
All attention has been focused on what antitumor immunity can do after it is activated by therapy and most previous efforts are focused on to activate antitumor immunity regardless whether antitumor immunity in a case is pre-existing or not. By the general rule of immunology, to activate a pre-existing immunity is a much easier task than to initiate a primary response. This should also be true in the case of antitumor immunity, but this limitation is often ignored. The role of concomitant antitumor immunity during tumor-bearing state can be thoroughly analyzed in animal tumor models because it is possible to manipulate the levels of antitumor immunity in animal through various means to remove immunity, thus creating a direct comparison between tumor growth with or without the influence of antitumor immunity. For example, T cells can be deleted through thymectomy, sublethal radiation and antibody-mediated cell phagocytosis (ADCP) and cell cytotoxicity (ADCC).
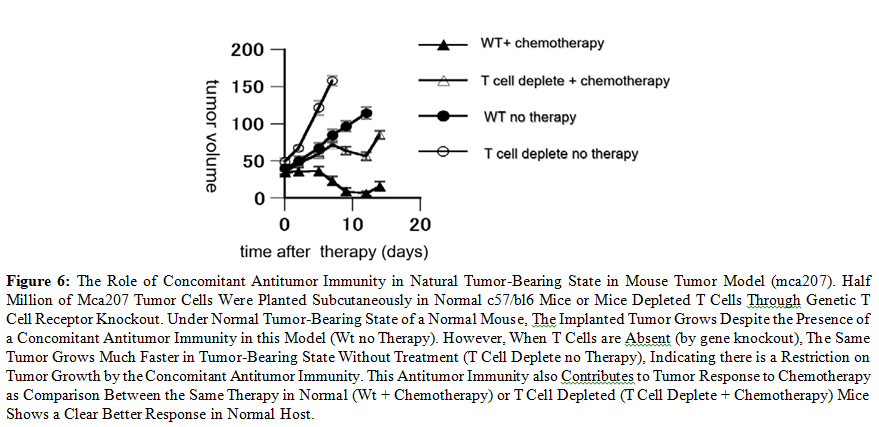
Under such circumstances, it could be seen that the presence of a steady state antitumor immunity does not prevent tumor progression if the immunity is initiated after establishment of tumor lesion. But the presence of such immunity can slow down tumor growth rate and make chemotherapy more effective (Figure6). Furthermore, with the presence of antitumor immunity (concomitant immunity), establishment of the same tumor at distant location by implant is prevented, but this prevention is abolished if T cells are depleted, indicating this is the function of antitumor immunity [8]. Since spread of animal tumors is often difficult to witness in the period of a few months, implant of tumor cells at distant location is used to mimic metastasis [8].
Despite these clear observations from animal tumor studies, similar manipulation in human patients is not possible for obvious ethical reasons. It is therefore difficult to “prove” the role of antitumor immunity in a natural tumor-bearing state in a patient. It is probably due to this lack of demonstration that clinicians are still ignorant of the anti-growth and anti-metastasis function of antitumor immunity in a given cancer case.
In this regard, the recently developed ICB antibody therapy offered an opportunity to obtain such a direct comparison in cancer patients due to the mis-understanding of the true mechanism behind this therapy. The current mechanism of ICB therapy adapted by the medical society is so-called “blocking model”[26]. In this model, tumor-expressed immune checkpoint molecule such as PD-L1 negatively regulate activated antitumor T cells through interaction with the counterpart ligand such as PD1 expressed on the surface of T cells.
This interaction results in the down regulation of T cell function against tumor. Antibodies to either PD1 or PD-L1 may block this interaction thus preventing the negative regulation to take place. Although this is the prevailing view on the mechanism of ICB therapy, this proposed mechanism lacks direct evidence in vivo and several critical observations associated with the clinical use of the ICB antibodies cannot be explained by this mechanism. For example, majority (>50%) of patients experience accelerated tumor progression immediately after the use of ICB antibodies (our unpublished observation). In some of these cases, antitumor effects are observed after a lagging period of 1-2 month, while in other patients, hyper-progression with explosive tumor growth of existing and new metastases leading to rapid death take place [20].
This temporary or persistent acceleration of tumor progression following ICB antibodies cannot be explained by the currently adapted blocking model. On the other hand, from many years of animal studies, loss of antitumor immunity will cause accelerated tumor progression including the ability to suppress new metastasis. Based on these considerations and observations, we have put forward another mechanism to explain ICB antibody therapy called depletion model [19].
According to this model, the temporary and persistent tumor progression following ICB antibody treatment is the result of partial or complete T cell depletion by antibodies. Although antibody producers have claimed that their antibodies have modified Fc sequences that reduce ADCP or ADCC-mediated antigen depletion, in vivo observation of rapid loss of T cells following antibody administration does not support their claim (and our unpublished observation) [28]. If indeed this is taking place in vivo, a patient with concomitant antitumor immunity may experience depletion of their antitumor T cells if these cells express antibody target molecule (for example, PD1) and are located in the interstitial space easily accessible by ICB antibody.
The consequence would be loss of tumor control and unchecked progression of the tumor. In the case of a highly malignant tumor with high ability to disseminate and establish metastases, a hyper- progression takes place. The degrees of tumor control before and after ICB antibody thus create a comparison to demonstrate the critical role of antitumor immunity under steady tumor-bearing state. As mentioned above, anyone who witness such comparison should have a life-time impression.
By this comparison, we now know that like in the animal tumor models, concomitant antitumor immunity in cancer patient is critical for controlling the progression of primary tumor and for prevention of new metastases. Like in the case of animal models, this immunity is often not strong enough to eliminate pre-existing tumors, but is able to suppress the growth rate of these tumors. Not only that, once this immunity is established, new metastasis is prevented. This is a very useful rule to gauge the presence of antitumor immunity in a given case without obtaining tumor samples for direct looking for T cells and antitumor functions in these samples. In many so-called pseudo-stages IV cases, we can see the co-existence of distant metastasis and antitumor immunity. The reason for their co-existence is the sequence of establishment: the metastases we saw were established before the establishment of antitumor immunity. In these cases, we do not see continued establishment of new metastases because the concomitant antitumor immunity is able to prevent the establishment of new metastasis. The management of these cases should be totally different from that for the real stage IV case in which there is no antitumor immunity established, because once the established metastases are eliminated by other means (for example radiation therapy), the primary tumor can be surgically removed to create a tumor-free situation. The residual antitumor immunity then will provide protection against recurrence by new metastasis. In the past 9 years, we have repeatedly carried out this strategy and obtained clinical cures in over a dozen cases (to be published elsewhere).
In light of the critical role of concomitant antitumor immunity in cancer patients, any treatment selection should take the status of their antitumor immunity into consideration. In essence, due to the critical role of antitumor immunity in almost all tumor-reductive therapies, the impact of any clinical intervention on the status of antitumor immunity should be considered before such intervention is applied [24]. For example, the effect of tumor removal by surgery on the residual immunity may cause the decay of immunity and the gradual loss of immune protection against metastasis [8]. The selection for surgical treatment therefore should fully consider this negative impact and should only be carried out when antitumor immunity is strong enough to provide adequate post-surgery protection against recurrence. Any manipulation before surgery that can increase the level of antitumor immunity should be considered because such manipulation will likely extend the protection period by immunity after surgery [8]. Neo-adjuvant chemotherapy is such a manipulate that elevates the levels of a pre-existing antitumor immunity, thus helps to extend the recurrence-free period after removal of primary cancer [8]. The impact of radiation may be both activation and inhibition of antitumor immunity because it causes release of tumor antigen through killing tumor cells and it may damage tumor-infiltrating T cells as well. Depend on the status of antitumor immunity and distribution, careful selection of radiation dose schedule can maximize the benefit while spare the damage of antitumor immunity.
In summary, antitumor immunity is the most critical factor determining the survival of cancer patients once the malignancy of a tumor is confirmed. This fact is known for over half century by tumor immunologists working with animal tumor models, but has been systematically ignored by cancer clinicians until the power of antitumor immunity in human cancer patients has been demonstrated by the ICB therapy in the past few years. Even so, the role of antitumor immunity in a naturally tumor bearing state (or in a concomitant state) remains unknown to most clinicians and the status of antitumor immunity still is not a consideration for therapy selection. In contrast, we have begun our individualized management of cancer patient almost a decade ago and our continued focus on the status of antitumor immunity in each patient has produced significant survival benefit for a group of late-stage and recurrent lung cancer patients during this time [29].
Here, we have described five ovarian cancer cases to demonstrate the First Law of TANLUN, i.e., once the malignancy of a tumor is confirmed, the status of the concomitant antitumor immunity is the most influencing factor determining the survival of the patient (for a more detailed view of TANLUN’s cancer management system, visit our web site at:www.tanlunforcancer,net). Because malignant tumor with high potential of metastasis is the reason that cancer is a difficult disease to manage, to keep the First Law of TANLUN in mind while managing difficult cancer cases will help clinicians to improve effectiveness in every case. To do so requires the awareness of the presence and roles of antitumor immunity in each case. This writing intends to raise that awareness in cancer clinicians.
References
1. Håkansson, L., Adell, G., Boeryd, B., Sjögren, F., & Sjödahl, R. (1997). Infiltration of mononuclear inflammatory cells into primary colorectal carcinomas: an immunohistological analysis. British journal of cancer, 75(3), 374-380.
2. Ropponen, K. M., Eskelinen, M. J., Lipponen, P. K., Alhava, E., & Kosma, V. M. (1997). Prognostic value of tumourâ? infiltrating lymphocytes (TILs) in colorectal cancer. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland, 182(3), 318-324.
3. Zhang, L., Conejo-Garcia, J. R., Katsaros, D., Gimotty, P. A., Massobrio, M., Regnani, G., ... & Coukos, G. (2003). Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New England journal of medicine, 348(3), 203-213.
4. Khan, H., Pillarisetty, V. G., & Katz, S. C. (2014). The prognostic value of liver tumor T cell infiltrates. Journal of Surgical Research, 191(1), 189-195.
5. Lee, H. E., Chae, S. W., Lee, Y. J., Kim, M. A., Lee, H. S., Lee, B. L., & Kim, W. H. (2008). Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. British journal of cancer, 99(10), 1704-1711.
6. Tewari, N., Zaitoun, A. M., Arora, A., Madhusudan, S., Ilyas, M., & Lobo, D. N. (2013). The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC cancer, 13, 1-10.
7. Ibrahim, E. M., Al-Foheidi, M. E., Al-Mansour, M. M., & Kazkaz, G. A. (2014). The prognostic value of tumor- infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast cancer research and treatment, 148, 467-476.
8. Chen, X., Wang, Q., Tsung, K., Antitumor Immunity as Determining Factor for Prevention of Post-Surgery Cancer Recurrence. Journal of Clinical & Experimental Immunology, 2020. 5(1): p. 1-11.
9. Gross, L. (1943). Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer research, 3(5), 326-333.
10. Foley, E. J. (1953).Antigenic properties of methylcholanthrene- induced tumors in mice of the strain of origin. Cancer research, 13(12), 835-837.
11. Prehn, R. T., & Main, J. M. (1957). Immunity to methylcholanthrene-induced sarcomas. Journal of the National Cancer Institute, 18(6), 769-778.
12. Deckers, P. J., Edgerton, B. W., Thomas, B. S., & Pilch, Y. H. (1971). The adoptive transfer of concomitant immunity to murine tumor isografts with spleen cells from tumor-bearing animals. Cancer Research, 31(6), 734-742.
13. North, R. J. (1984). The therapeutic significance of concomitant antitumor immunity: II. Passive transfer of concomitant immunity with Ly-1+ 2− T cells primes established tumors in T cell-deficient recipients for endotoxin-induced regression. Cancer Immunology, Immunotherapy, 18, 75-79.
14. North, R. J. (1984). The therapeutic significance of concomitant antitumor immunity: I. LY-1− 2+ T cells from mice with a progressive tumor can cause regression of an established tumor in γ-irradiated recipients. Cancer Immunology, Immunotherapy, 18, 69-74.
15. North, R. J., & Bursuker, I. S. I. A. (1984). Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+ 2-suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. The Journal of experimental medicine, 159(5), 1295-1311.
16. Prehn, R. T. (1972). The immune reaction as a stimulator of tumor growth. Science, 176(4031), 170-171.
17. Prehn, R. T. (1993). Tumor immunogenicity: how far can it be pushed?. Proceedings of the National Academy of Sciences, 90(10), 4332-4333.
18. Prehn, R. T. (2005). On the nature of cancer and why anticancer vaccines don't work. Cancer Cell International, 5, 1-5.
19. Tsung, K., & Xu, Z. (2022). The Blocking vs. Depletion Model of Immune Checkpoint Inhibitor Therapy for Cancer. Immunome Research, 18(2), 1-8.
20. Kim, J. Y., Lee, K. H., Kang, J., Borcoman, E., Saada- Bouzid, E., Kronbichler, A., ... & Gamerith, G. (2019). Hyperprogressive disease during anti-PD-1 (PDCD1)/PD- L1 (CD274) therapy: a systematic review and meta-analysis. Cancers, 11(11), 1699.
21. Tusung, K. (2019). The Presence of Autonomous and Non- Autonomous Replication in Cancer and their Connection through Inflammation: Lessons from one Lung Cancer Case. Immunome Research, 15(3), 1-7.
22. Tsung K, Z.X.a.Z.H., Existence and Significance of Non- Autonomous Cancer Replication for Clinical Management of Cancer: Observations and Hints from Ovarian Cancer Cases. Journal of Pathology Research Review and Reports, 2020. 2(2): p. 1-8.
23. Zhang, L., Feng, D., Yu, L. X., Tsung, K., & Norton, J.A. (2013). Preexisting antitumor immunity augments the antitumor effects of chemotherapy. Cancer Immunology, Immunotherapy, 62, 1061-1071.
24. Guo, H., & Tsung, K. (2017). Tumor reductive therapies and antitumor immunity. Oncotarget, 8(33), 55736.
25. Chen, S., Crabill, G. A., Pritchard, T. S., McMiller, T. L., Wei, P., Pardoll, D. M., ... & Topalian, S. L. (2019). Mechanisms regulating PD-L1 expression on tumor and immune cells. Journal for immunotherapy of cancer, 7, 1-12.
26. Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature reviews cancer, 12(4), 252- 264.
27. Tsung K, Z.X., Zhang Hui, and TANLUN Clinical Research Participants Group, Understanding and Management of Cancer Recurrence. Medical & Clinical Research, 2023. 9(1): p. 1-12.
28. Brahmer, J. R., Drake, C. G., Wollner, I., Powderly, J. D., Picus, J., Sharfman, W. H., ... & Topalian, S. L. (2010). Phase I study of single-agent anti–programmed death-1 (MDX- 1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology, 28(19), 3167-3175.
29. Tsung K, Z.X., Zhang Hui, and TANLUN Clinical Research Participants Group, On the Individualized Cancer Management. Medical & Clinical Research, 2023. 8(10): p. 1-9.


