Research Article - (2025) Volume 10, Issue 1
Predominance of Catalase Gene Mutations in Type 2 Diabetes Mellitus High-Risk Population
2Dr. Zafar H. Zaidi Center for Proteomics, University of Karachi, Karachi 75270, Pakistan
3Baqai Institute of Diabetes and Endocrinology, Karachi 74600, Karachi,, Pakistan
Received Date: Jan 02, 2025 / Accepted Date: Feb 03, 2025 / Published Date: Feb 12, 2025
Copyright: ©�2025 Syeda Nuzhat Nawab, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Nawab, S. N., Kafeel, S., Hashim, Z., Fawwad. A. (2025). Predominance of Catalase Gene Mutations in Type 2 Diabetes Mellitus High-Risk Population. Int J Diabetes Metab Disord, 10(1), 01-08.
Abstract
Objective: To determine the-role of CAT -21-A/T (rs7943316) and -262 C/T (rs1001179) genetic/variants/in the susceptibility of type 2 diabetes mellitus (T2DM). The study also explored the likelihood of clinical and anthropometric-associated variations in T2DM.
Methods: This case-control study comprised of total n=400 subjects categorized into two/groups: T2DM (n=200) and controls (n=200). Genotyping of variants/was-carried-out-by allele-specific (AS) and RFLP-PCR/based/strategies.
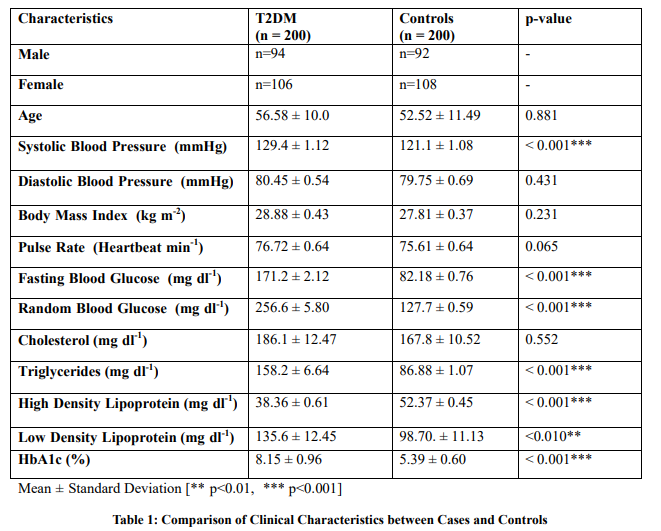
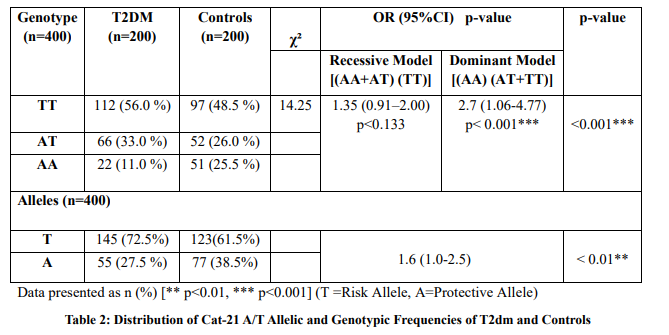
Results: Distribution of genotypes revealed significant differences in both variants of CAT gene promotor. In CAT -21 A/T variant, the frequency of the mutant T/T genotype was higher in the T2DM group (56.0%) as controls (48.5%). While, in CAT -262 C/T variant, the frequency of heterozygous genotype C/T was significantly higher in diseased patients (64%)/ compared/to/controls (33%). Statistical association analysis demonstrated the significant implication of both CAT variants in the pathogenicity of T2DM (p<0.001). A significantly higher trend of systolic blood pressure, glucose, triglycerides, and LDL cholesterol levels was observed in T2DM patients (p<0.01). Whereas, plasma levels of HDL cholesterol were found to be within range in controls (p<0.001).
Conclusions: CAT variants may be regarded as potential biomarkers for the prognosis of T2DM. CAT (rs7943316) variant was involved in the modulation of risk associated with T2DM. While CAT (rs1001179) variant was involved in the development of resistance against the predisposition of T2DM.
Keywords
Catalase, clinical, genotypes, polymorphism, type 2 diabetes mellitus
Introduction
The prevalence of T2DM has frequently been increasing all around the world. The global prevalence estimated by the international diabetes federation (IDF) Atlas, 2019 reported a 9.3% rate for type 2 diabetes affecting around 463 million subjects that might be enhanced up to 10.2% by 2030 and expected to be 10.9% by 2045 [1]. In Pakistan, the prevalence of T2DM is 17.1% projected by 9th edition of IDF [2]. T2DM is one of the common diseases in the world and its complications have become a major cause of death and disabilities [3,4]. Genetic predisposition, high caloric diets, and sedentary lifestyle are the foremost causes for the onset of disease condition [5].
In addition, oxidative stress has also been considered as a major pathogenic factor for the development of the complications [6,7]. Oxidative stress is produced due to increased generation of reactive oxygen species (ROS). These free radicals are synthesized in the body during normal metabolism. However, the diseased condition is often associated with over production of ROS. The antioxidant enzyme system is available in the body that protects against the damaging effect of ROS. Oxidative stress is initiated when the level of ROS becomes amplified which in turn diminishes the antioxidant defense mechanism [8]. It is reported that genes associated with antioxidant enzymes are susceptible to developing genetic variations hence impeding the enzyme activity and alter its expression [9,10]. Catalase is considered as one of the powerful antioxidant intracellular enzymes. It mainly takes part in the decomposition of hydrogen peroxides (H2 O2 ) into the simplest molecular forms of water and oxygen molecules [11]. CAT gene is located at chromosome 11 p13. There are many genetic variants that have been frequently reported in the human CAT gene [9].
CAT -21A/T (rs7943316) and 262C/T (rs1001179) are the polymorphisms which are located in the promotor region. CAT -21 A/T and CAT-262 C/T are the genetic variations which could exert detrimental effects on its catalytic activity due to inappropriate binding of-transcription-factors which leads to alter gene expression [12]. Eventually, it results in a high concentration of H2 O2 that promotes oxidative stress and hence oxidative damage [13]. Since oxidative stress not only increases the developing risk of diabetes but also plays a crucial role in the onset of late diabetic complications [6]. Several studies have reported that the intensity of oxidative damage is caused due to deficiency of antioxidant enzymes such as SOD, CAT, and GPX1. An enzyme of CAT can efficiently decay 2–20 million H2 O2 molecules in a second [12,13]. The genetic variations in its gene may compromise the pronounced role and efficacy of the CAT enzyme which led to enhanced susceptibility to oxidative stress-induced diseases such as T2DM. Thus, the role of-CAT genetic variations of -21 A/T (rs7943316) and-262 C/T (rs1001179) in promotor region was investigated in association with the risk of developing the susceptibility of T2DM.
Materials and Methods
This case control study comprised of total n=400 subjects categorized into two groups: T2DM (n=200) and controls (n=200). Samples were collected from the outpatient department-(OPD) of Baqai Institute of Diabetology and Endocrinology (BIDE). Prior approvals from the respective ethical review board and committees of the University of Karachi and BIDE were obtained before the commencement of the research project. The informed consent was taken from all participants. The demographic data was confidentially recorded using a purposely designed questionnaire after pilot testing. Blood samples were drawn from cephalic vein in Ethylene Diamine Tetra Acetic-(EDTA) vacutainers for DNA extraction and HbA1c testing, in fluoride-vacutainers for estimations of glucose level and in gel vacutainers for the biochemical-testing. After collection, gel vacutainers were centrifuged at 3500 rpm for 20 minutes, serum samples were aliquoted and stored at-20 °C for further analysis.
DNA Extraction
Extraction of DNA was performed by following the standardlab protocol of salting-out [14]. The quantity of he DNA for total n=400 samples were estimated by measuring 260 280 ratio and optical density (OD) of 1.7 to 1.8 using Nanodrop Analyzer (IMPLEN Nano Photometer® P-Class, Germany). Though, the quality of DNA was analyzed by gel electrophoresis.
Genotyping
Extracted DNA samples were used for the genotyping of CAT -21 A/T (rs7943316) genetic-variant-by PCR-RFLP-based method. Total 50 µl of total reaction mixture of PCR was prepared which-consist of 150 ng of genomic DNA, 0.5 µM of forward 5′AATCAGAAGGCAGTCCTCCC-3′ and reverse -primers 5′ TCGGGGAGCACAGAGTGTAC 3′, 0.2 mM of dNTPs, 1.5mMof MgCl2 and 5 units of Taq DNA polymerase-added in 1X-PCR buffer (Thermo Fisher Scientific). The program used for the amplification of-targeted region consists of-initiation at-95°C for 5 minutes, -35 cycles of denaturation at 95°C for 35 seconds, annealing at 62° C for 40seconds and extension at 72°C for 30 seconds. Then final elongation was performed at 72°C extension for 7 minutes. The detection of CAT-21 A/T (rs7943316) variant was undertaken using10U of Hinf1 restriction endonuclease enzyme selected by NEB cutter. It was used for the digestion of the PCR product, which was assayed on 1.5% agarose gel to observe the banding patterns of its genotypes.
CAT -262C/T (rs1001179) polymorphism was genotyped by using allele-specific PCR based method. For a single reaction, a total of 25 µl of total reaction mixture of PCR was prepared which consist of 150 ng of genomic DNA, total reaction mixture of PCR 0.5 µM of forward-1 (F1) primer 5’-GCCCTGGGTTCGGCTATC-3’ for C allele or Forward-2 (F2) primer 5’-GCCCTGGGTTCGGCTATT- -3’for T allele and reverse primers5’-GTTTGCTGTGCAGAACACT-3’,0.4 mM of dNTPs, 2.0mM of MgCl2 and 2.5 units of Taq DNA polymerase (Thermo Fisher Scientific). was added in 1X-PCR buffer. The program used for the targeted CAT -262C T (rs1001179) polymorphism consists of-initiation at 95°C for 5 minutes, 30 cycles of denaturation at 94°C for 45 seconds, annealing at-61°C for 40 seconds, and extension at 72°C for 45 seconds. Final elongation was performed at 72°C for an extension of 5 minutes followed by product analysis of 340 bp for C or T allele on 2% (W/V) agarose gel.
Statistical and Bioinformatic Analysis
Interpretation of biochemical parameters and genotypic data was performed -using a software statistical package for the social sciences (SPSS) version 16.0. The average was compared to demonstrate the difference between the groups of cases and control using an independent sample t-test. The Chi-square and odds ratio statistics were interpreted to investigate the association of genotypic distribution for the two variants with the disease. Dominant and recessive models-for CAT genetic variations were also calculated to infer their overall risk from the combined impact of -21 A/T and -262 C/T genotypes respectively in diverse states.
Results
A total of n=400 subjects were participated in this study. The comparison of clinical characteristics between T2DM and control groups is shown in Table 1. It reflected the relationship between these variables with the pathogenicity of the disease. Furthermore, the distribution of allelic and genotypic frequency along with the association of CAT -21 A/T (rs7943316) variant/is demonstrated in Table 2, while the inference of the CAT -262 C/T (rs1001179) variant is depicted in Table 3. The genetic analysis of CAT -21 A/T (rs7943316) and CAT -262 C/T (rs1001179) variants described the pattern of genotypes in T2DM and control groups. The homozygosity and heterozygosity of CAT -21A/T and CAT -262 C/T alleles were shown by allele-specific PCR fragments in Figures 1 & 2 respectively.



Bands of 340 bp for C and T alleles of the same DNA samples showed in each column. Marker (M): 100bp, Lanes: 4-6, 8, 12 & 14 denoted the genotype of homozygous CC; lanes 1-3, 9, 10 & 13 represented the genotype of heterozygous CT, while lanes 7 & 11 depicted the genotype of homozygous TT [15-18].
Discussion
The current study examined the association of CAT variants -21 A/T (rs7943316) and-262 C/T (rs1001179) with/the/susceptibility of/T2DM. A high frequency of homozygous TT genotype of CAT -21 A/T variant was observed in the disease group as compared to controls (56.0 % vs. 48.5 %). Chi-square demonstrated the significant difference in the distribution of CAT -21 A/T (rs7943316) variant genotypes between the groups of cases and controls (p<0.001). Furthermore, a significantly higher frequency of the mutant T allele elevated the risk of T2DM by 1.6 times as compared to wild type A allele (p<0.01). The genotypic frequency of the CAT -21 A/T variant was found to be significantly different between the hypothetical dominant and recessive genotypic models (p< 0.05). A significant association of genotypes was found in the dominant state for the T allele in T2DM patients (p<0.001). Though, the TT genotype was more consistent in T2DM patients and statistically higher in comparison with controls. The odds ratio of the dominant model [(AA) (TA+TT)] indicated that the TT genotype of CAT (-21 A/T) variant may increase 2.7 folds risk for the onset of type 2 diabetes (Table 2). A previous study has revealed that in the CAT -21 A/T variant, the TT genotype plays a prompting role with significantly higher frequency in the onset of diabetes as a possible genetic risk factor [19]. An earlier study indicated that genetic variants reported in the promoter region of the CAT gene were involved in reducing the expression of a gene [20]. Among them, it was revealed that the TT genotypic frequency of CAT -21 A/T (rs7943316) variant has 80% frequency in the subjects of T2DM with the diminished enzymatic activity of CAT. Increased risk of cerebral stroke and hypertension was observed in a study related to the association of CAT -21 A/T variant [21]. Whereas, Yeh et al., reported that there was an insignificant association between CAT variants of promotor region and susceptibility of coronary artery disease [22]. However, limited literature was found for the investigation of genetic models of-both CAT -21 A/T and -262 C/T variants in the predisposition toT2DM [23].
The present research also explored the distribution of another genetic variant CAT -262 C/T (rs1001179) which was also reported in the promotor region [12]. CAT -262 C/T variant has shown significant association against the susceptibility of T2DM. The distribution of CAT -262 C/T variant genotypes explained that CC genotypic frequency is significantly higher in the diseased group (64 %) than those of controls (33 %). Whereas, the frequency of CT genotype was inferred to be reduced in cases (27.5 %) in comparison to controls (47.5 %). Correspondingly, the TT genotypic frequency was also found to be lowered in the disease group (8.5 %) concerning controls (19.5 %). The chi-square test revealed a significant difference in the genotypic distribution of the CAT -262 C/T variant between the groups of cases and controls (χ²= 38.71, p<0.001). However, the outcomes showed that C allelic frequency was a significantly higher in T2DM subjects (77.5 %) whereas allelic frequency of T allele was higher in controls (43%). Henceforth, it suggested a predisposing influence of the C allele, while the T allele was found to implement a protective impact with an odds ratio of 0.384 to the susceptibility of T2DM. The genetic models were evaluated to infer the combined effect of genotypes. The odds ratio of the recessive model recommended that the T allele produce 0.42 times higher risk than the C allele for the onset of type 2 diabetes mellitus (p<0.001) (Table 3). This variant was reported to express its functional association with the expression of the CAT enzyme. A significantly elevated level of CAT-enzyme was previously reported in the presence of homozygous TT genotype of CAT -262 C/T variant [24]. However, the enzyme was observed to be significantly reduced with the CC homozygous genotype which ultimately reflected its significant role in declining the expression of the CAT gene [25]. Another study conducted on T2DM patients also suggested the imperative role of the T allele of CAT -262 C/T variant which provides resistance against the induction and progression of oxidative stress and its associated ailments [26]. Formerly, the -262 C/T genetic variant of the CAT gene has also been found to exert a significant impact in the modulation of risk for autoimmune diabetes and or related complications [27]. Apart from diabetes, a study reported the significant role of the CAT-262 variant in demolishing the susceptibility of breast cancer [28]. However, no evidence of association was reported for the susceptibility of nephropathy and retinopathy in type 2 diabetes [29]. Catalase promoter region variants-of -21A/T- (rs7943316) and - 262 C/T - (rs1001179) -may cause an effect on the susceptibility of oxidative stress-related diseases which might involve in the alterations of transcription factors PAX-6-and-STAT 4 in the presence of A and T allele [30]. The level of CAT mRNA was significantly affected by the occurrence of CAT -21 A/T and -262 C/T genetic variants found in the promotor region of the CAT gene [31]. Additionally, the levels of FBG, RBG, and HbA1c in the blood, and TG, HDL and LDL in the serum illustrates the significant difference between T2DM patients and controls, which demonstrated an association with the predisposition of the disease. Nevertheless, the variables of BMI, pulse rate, DBP, and total cholesterol reflected no significant differences between the study groups of T2DM and controls (Table 1).
The investigation of CAT variants showed significant genetic associations with the risk and pathogenicity and could be considered as a baseline for developing advances as a worthwhile approach in screening, resistance, and treatment strategies of T2DM. Hence, it is essential to consider the limitations before drawing the actual conclusions of this study. Firstly, the correlation between the mutant allele of two CAT variants with the expressional variability of CAT enzyme is important to reveal its influence in developing the pathogenicity of T2DM that is not explored to the studied variant. Secondly, the present study consists of a conserved group of samples for a population-based study. It is essential to undergo large-scale analysis for revealing interpretations with more accuracy and efficiency.
Conclusion
The findings revealed that two genetic variants of the CAT gene showed significant association with the pathogenesis of T2DM. The genetic variant of -21 A/T (rs7943316) in the promotor region of the CAT enzyme gene might play an effective role in the modulation of high risk for T2DM. Whereas, the CAT -262 C/T (rs1001179) variant inferred a defensive role against the susceptibility of the disease. Hence, both variants of CAT -21 A/T/(rs7943316) and -262 C/T (rs1001179) could be regarded as a prognostic antioxidant biomarkers in determining the predisposition of T2DM.
Highlights
• Oxidative stress is regarded as a major pathogenic factor for the development of diabetes and its associated complications.
• The antioxidant enzyme catalase diffuses the damaging effect of H2 O2 which contributes to developing oxidative stress.
• The Catalase enzyme gene is susceptible to developing genetic variations which cause the modulation of its regulation and expression in disease conditions.
• CAT -21A/T (rs7943316) polymorphism depicted a significant association with a higher risk for T2DM.
• CAT -262C/T (rs1001179) polymorphism inferred a significant protective role in response to the progression of T2DM.
• CAT -21 A/T and CAT -262 C/T can be considered as a potential biomarker in determination of susceptibility for T2DM patients.
Acknowledgements
Acknowledgments The authors acknowledge the support research department of the Baqai Institute of Diabetology and Endocrinology (BIDE) for their active participation and collaboration.
References
- Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., ... & IDF Diabetes Atlas Committee. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice, 157, 107843.
- Williams, R., Karuranga, S., Malanda, B., Saeedi, P., Basit, A., Besançon, S., ... & Colagiuri, S. (2020). Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice, 162, 108072.
- Olbeci, O., Celik, A., & Jone, R. (2020). The The Barriers and Facilitators of Physical Activity Participation Among People Living with Type 2 Diabetes. Social Science Protocols, 3, 1-16.
- Saklayen, M. G. (2018). The global epidemic of the metabolic syndrome. Current hypertension reports, 20(2), 1-8.
- Qi, Q., Meigs, J. B., Rexrode, K. M., Hu, F. B., & Qi, L. (2013).Diabetes genetic predisposition score and cardiovascular complications among patients with type 2 diabetes. Diabetes care, 36(3), 737-739.
- Halim, M., & Halim, A. (2019). The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes & metabolic syndrome: clinical research & reviews, 13(2), 1165-1172.
- Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F., & Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell death & disease, 9(2), 119.
- Liu, Z., Ren, Z., Zhang, J., Chuang, C. C., Kandaswamy, E., Zhou, T., & Zuo, L. (2018). Role of ROS and nutritional antioxidants in human diseases. Frontiers in physiology, 9, 360203.
- Wang, C. D., Sun, Y., Chen, N., Huang, L., Huang, J. W., Zhu, M., ... & Ji, Y. L. (2016). The role of catalase C262T gene polymorphism in the susceptibility and survival of cancers. Scientific reports, 6(1), 26973.
- Sharifi-Rad, M., Anil Kumar, N. V., Zucca, P., Varoni, E. M., Dini, L., Panzarini, E., ... & Sharifi-Rad, J. (2020). Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Frontiers in physiology, 11, 694.
- Lismont, C., Revenco, I., & Fransen, M. (2019). Peroxisomal hydrogen peroxide metabolism and signaling in health and disease. International Journal of Molecular Sciences, 20(15), 3673.
- Hernández-Guerrero, C., Parra-Carriedo, A., Ruiz-de- Santiago, D., Galicia-Castillo, O., Buenrostro-Jáuregui, M., & Díaz-Gutiérrez, C. (2018). Genetic polymorphisms of antioxidant enzymes CAT and SOD affect the outcome of clinical, biochemical, and anthropometric variables in people with obesity under a dietary intervention. Genes & nutrition, 13, 1-10.
- Saify, K. (2016). Genetic polymorphisms in the promoter region of catalase gene, creates new potential PAX-6 and STAT4 response elements. Molecular Biology Research Communications, 5(2), 97.
- Thakur, P., Kumar, A., & Kumar, A. (2018). Targeting oxidative stress through antioxidants in diabetes mellitus. Journal of drug targeting, 26(9), 766-776.
- MWer, S., Dykes, D., & Polesky, H. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids res, 16(3), 1215.
- Yassaee, F. (2018). A18061 Comparison of CAT-21A/T gene polymorphism in women with preeclampsia and control group. Journal of Hypertension, 36, e256.
- Khodayari, S., Salehi, Z., Fakhrieh Asl, S., Aminian, K., Mirzaei Gisomi, N., & Torabi Dalivandan, S. (2013). Catalase gene Câ?262T polymorphism: importance in ulcerative colitis. Journal of gastroenterology and hepatology, 28(5), 819-822.
- Yong, Y. O. N. G., & He, L. (2005). SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell research, 15(2), 97.
- Alipour, M., Naeimi, S., Moghanibashi, M. M., & Mahmmodian, Z. (2020). Association between Catalase Gene (CAT-262 C/T) Polymorphism and Preeclampsia in the South of Iran. Journal of Ilam University of Medical Sciences, 28(2), 11-20.
- Dhanapal K, Selvan N, Dhananjeyan, V. A study on catalase activity and its genetic variant in diabetes mellitus patients. Online J Biol Sci. 2010;10:653-657.
- Zhou, X. F., Cui, J., DeStefano, A. L., Chazaro, I., Farrer, L. A., Manolis, A. J., ... & Baldwin, C. T. (2005). Polymorphisms in the promoter region of catalase gene and essential hypertension. Disease markers, 21(1), 3-7.
- Yeh, H. L., Kuo, L. T., Sung, F. C., & Yeh, C. C. (2018). Association between polymorphisms of antioxidant gene(MnSOD, CAT, and GPx1) and risk of coronary artery disease. BioMed research international, 2018(1), 5086869.
- Ghattas, M. H., & Abo-Elmatty, D. M. (2012). Association of polymorphic markers of the catalase and superoxide dismutase genes with type 2 diabetes mellitus. DNA and cell biology, 31(11), 1598-1603.
- DoÄ?an, A., ÖzÅ?ensoy, Y., & Türker, F. S. (2019). MnSOD, CAT and GPx-3 genetic polymorphisms in coronary artery disease. Molecular biology reports, 46, 841-845.
- Góth, L., Rass, P., & Páy, A. (2004). Catalase enzyme mutations and their association with diseases. Molecular Diagnosis, 8, 141-149.
- Nandi, A., Yan, L. J., Jana, C. K., & Das, N. (2019). Role of catalase in oxidative stressâ?and ageâ?associated degenerative diseases. Oxidative medicine and cellular longevity, 2019(1), 9613090.
- Chistiakov, D. A., Zotova, E. V., Savost'anov, K. V., Bursa, T. R., Galeev, I. V., Strokov, I. A., & Nosikov, V. V. (2006). The 262T> C promoter polymorphism of the catalase gene isassociated with diabetic neuropathy in type 1 diabetic Russian patients. Diabetes & metabolism, 32(1), 63-68.
- Saadat, M., & Saadat, S. (2015). Genetic polymorphism of CAT C-262 T and susceptibility to breast cancer, a case– control study and meta-analysis of the literatures. Pathology & Oncology Research, 21, 433-437.
- dos Santos, K. G., Canani, L. H., Gross, J. L., Tschiedel, B., Souto, K. E. P., & Roisenberg, I. (2006). The catalase– 262C/T promoter polymorphism and diabetic complications in Caucasians with type 2 diabetes. Disease markers, 22(5-6), 355-359.
- Arpaci A, Yalın S, Ecevit H, Comelekoglu U, Mete T. Enzyme activity and genetic variants in patients with type II diabetes mellitus. Adv Clin Exp Med. 2020;29:1057-1063.
- Saify, K., Saadat, I., & Saadat, M. (2016). Influence of A-21T and C-262T genetic polymorphisms at the promoter region of the catalase (CAT) on gene expression. Environmental health and preventive medicine, 21, 382-386.



