Research Article - (2024) Volume 2, Issue 2
Efficacy of Intra-Articular Hyaluronic Acid Injections with INNORYOS 2.2% in Patients with Knee Osteoarthritis
2Practice of Orthopedics and Trauma Surgery, ├ľttingen, Germany
Received Date: Sep 09, 2024 / Accepted Date: Nov 13, 2024 / Published Date: Dec 09, 2024
Copyright: ©ę2024 Simon von Stengel, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Hofweber, L., Kemmler, W., N├╝rnberger, J., Stengel, S, V. (2024). Efficacy of Intra-Articular Hyaluronic Acid Injections with INNORYOS 2.2% in Patients with Knee Osteoarthritis. Japan J Med Sci, 2(2), 01-10.
Abstract
Introduction: We determined the effectiveness and safety of INNORYOS 2.2% hyaluronic acid (HA) containing 1.5% of niacinamide on knee pain, stiffness and function in people with knee osteoarthritis (OA) compared with an established agent. Further study objectives were to monitor side effects during the study period.
Methods: The 26-week prospective non-interventional open clinical trial tested on non-inferiority of INNORYOS 2.2% compared to an established HA product (Synvisc 0.8%, 2.0 ml). Briefly, 90 patients with knee OA (Kellgren-Lawrence grade I to III), 40-85 years old, were allocated randomly to the two groups. HA injections were carried out at baseline and after 1 and 2 weeks. Additional visits for data collection were made after 14 and 26 weeks. Changes in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), pain visual analogue scale (VAS), side effects and confounders were assessed.
Results: None of the 90 participants (65.0±10.5 years, BMI: 30.2±5.5 kg/m2 ) quit the study or was lost to follow-up. After 26 weeks, in both groups the WOMAC total score had developed positively with no significant between-group difference (-37.1% treatment vs -29.2% control). WOMAC pain and function subscores did not differ significantly between the groups, but WOMAC stiffness developed in favor of INNORYOS 2.2% group (p=.009). Pain, as assessed by VAS reduced to a similar extent in both groups (-35.2% treatment vs. -31% control). No adverse effects were observed or reported by the participants. No changes of co-medication with impact on the study outcomes were observed.
Conclusion: We did not determine significantly lower effects of INNORYOS 2.2% compared with the established, high molecular weight, cross-linked product Synvisc. In contrast, INNORYOS 2.2% tendentially revealed more favorable effect on all WOMAC categories (significant for “stiffness”). Thus, the present study provided further evidence for the effectiveness and safety of INNORYOS 2.2% in patients with early to advanced knee OA.
Keywords
Hyaluronic Acid, Innoryos 2.2%, Knee, Niacinamide, Osteoarthritis
Introduction
Osteoarthritis (OA) is a chronic, degenerative, and progressive disease of the joint [1]. Its prevalence has increased due to aging populations, rising rates of obesity and increased incidence of injuries. In 2019, there has been a 113% increase in the number of people affected since 1990 [2]. The knee is the most commonly affected joint, with a prevalence of 365 million cases [3]. As 73% of individuals with OA are over 55 years old, and the risk of operative complications increases with age, the development of effective conservative treatments remains crucial. Additionally, the Osteoarthritis Research Society International (OARSI) recommends a conservative approach over surgical management [2,4,5].
Several studies have demonstrated the efficacy and safety of hyaluronic acid (HA) preparations for intra-articular injection [6- 11]. To potentially enhance their efficacy in alleviating pain, joint stiffness, and improving mobility, HA preparation can be combined with other inflammation-modulating agents. Niacinamide, an amide of nicotinic acid, possesses anti-inflammatory properties and may positively influence degenerative processes in arthritic joints [12]. As the efficacy of oral niacinamide intake has already been investigated and shown to have promising properties in pain relief and side effects, this study aimed to test the efficacy and safety of intra-articular injection of HA in combination with niacinamide for the knee [12-14]. The investigational product, INNORYOS 2.2%, containing 1.5% of niacinamide, was compared with the standard preparation Synvisc in terms of pain relief, improvement of joint stiffness, and mobility.
Primary hypothesis: The average effect of INNORYOS 2.2% on Western Ontario and McMaster Universities Osteoarthritis Index (total WOMAC score) is not inferior to the control group provided with the standard agent (Synvisc 0.8%, 2.0 ml).
Secondary hypothesis: (a) Average positive change of at least 50% in WOMAC “pain”, “stiffness” and “function” subscales after INNORYOS 2.2% injection from baseline to final follow- up assessment (26 weeks) [6]. (b) Positive treatment effects1 on total WOMAC Score from baseline to final follow-up assessment (26 weeks) after INNORYOS 2.2% treatment in at least 75% of the participants (c) Positive treatment effects1 on WOMAC “pain” subscale two weeks after the first (baseline) INNORYOS 2.2% injection. Further study aims were monitoring of co-medication and adverse effects during the study period.
Methods
The present study is a prospective non-interventional open clinical trial with two study arms conducted in a parallel group design. Briefly, the study aimed to determine the effectiveness and safety of INNORYOS 2.2% (ALBOMED, Germany) on knee OA in adults 40-85 years old. The study was initiated, planned and conducted by ALBOMED GmbH (Schwarzenbruck, Germany) in close cooperation with the Orthopedics and Trauma Surgery Öttingen (Germany) and the Institute of Radiology, University Hospital Erlangen, Germany. The present study started in November 2021 and was conducted over 26 weeks. The ethics committee of the Bayerische Landesärztekammer approved the trial that fully complies with the Helsinki Declaration “Ethical Principles for Medical Research Involving Human Subjects” [15]. After receiving detailed information, all study participants gave their written informed consent.
Participants
Potential study participants were selected by the Orthopedics and Trauma Surgery Öttingen (Germany) applying the following eligibility criteria:
Inclusion Criteria:
• Patients who fulfill the clinical criteria of the American College of Rheumatology for joint OA and are considered suitable for viscosupplementation by the examiner (Jorgen Nürnberger)
• Primary joint OA
• Age between 40 and 85 years
• Kellgren-Lawrence grade (KL) I to III on standing anteroposterior view [16]
Exclusion Criteria
• Trauma or surgery to the affected knee in the last 6 months
• Viscosupplement treatment on the affected knee within the last 6 months
• Injection with steroidal preparations on the affected knee within the last 3 months
• Strong inflammation of the affected joint
• Skin irritation or infection at the injection site
• Known hypersensitivity to hyaluronic acid or other product components
• Known autoimmune disease or other relevant abnormal physiological condition
• Congenital or drug-induced blood clotting disorders, for example due to hemophilia or the use of anticoagulants such as Marcumar (Phenprocoumon) or Coumadin (Warfarin)
• Pregnancy or breastfeeding
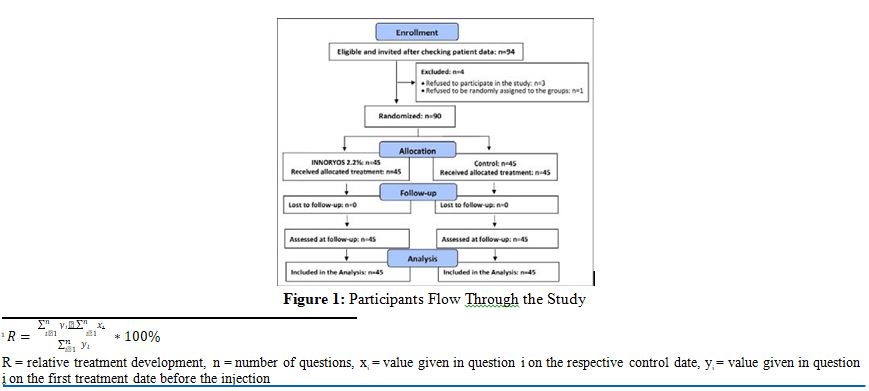
According to the sample size calculation, in summary 90 patients were selected and included in this prospective non-interventional open clinical trial (Fig. 1)
Blinding
The blinding strategy focused on outcome assessors and the statistician who were kept unaware of the participants’ group status (INNORYOS or control group (CG)) and were not allowed to ask, either.
Intervention
The participants were randomly assigned to one of the two groups by drawing lots. Both the intervention group and the control group were provided with an HA agent injected in their osteoarthritic knee at baseline and after 1 and 2 weeks (visit 1, 2 and 3), respectively. While the intervention group was treated with INNORYOS 2.2%, the CG was given the hyaluronic acid compound Synvisc. Follow- up visits were scheduled after 14 and 26 weeks (visit 4 and 5) (Tab.1).
|
Week |
baseline |
1 |
2 |
14 |
26 |
|
Visit number |
1 |
2 |
3 |
4 |
5 |
|
Injection |
X |
X |
X |
--- |
--- |
|
Outcome assessments |
X |
X |
X |
X |
X |
|
Adverse effects |
--- |
X |
X |
X |
X |
|
Confounders |
X |
X |
X |
X |
X |
Table 1: Brief Overview on INNORYOS 2.2% Trial
INNORYOS
The INNORYOS study arm was provided with INNORYOS 2.2%, 2.0 ml (ALBOMED GmbH (Schwarzenbruck, Germany)). The product is certified by HTCert (Certificate No: 2385C03210501). The injectable, colorless, and resorbable hydrogel was used in a dosage form of 2 ml packed in 2.25 ml sterile syringe, containing 2.2% sodium hyaluronate, for direct joint injection (Tab. 2). Two ml INNORYOS contains 1.5% of niacinamide. The selection of the injection technique was determined by the administering physician based on the specific anatomical considerations of the patient and their pain sensitivity.
The main active ingredient, linear hyaluronic acid obtained from bacterial fermentation, is known to alleviate the typical symptoms of cartilage degradation, such as pain, stiffness and movement restrictions [10, 17-19]. The compound contains niacinamide, which supports the main function by inhibiting the degeneration of the hyaluronate chain molecules [14].
Niacinamide is an important biomolecule that is involved in the redox reactions of metabolism. As a component of the coenzymes NAD+ and NADP+, it has antioxidant and anti-inflammatory properties which lead to a reduction of free radicals in the joint and therefore decreases oxidative stress [12].
|
Concentration |
2.2% Hyaluronic acid + 1.5% Niacinamide |
|
Molecular weight |
1.2-2.2 MDa |
|
Viscosity |
370 000 |
|
Volume |
2.0 ml |
|
Forms of therapy |
1 to 5 injections |
|
Storage |
2-25°C |
|
Shelf life |
42 month |
Table 2: INNORYOS 2.2% Datasheet
Control Group
The control group received an established OA product (Synvisc 0.8%, 2.0 ml). The positive effects and safety of Synvisc 0.8%, 2.0 ml have been tested in several studies [20-26].
The sodium hyaluronate contained (hylan G-F 20) is a high molecular weight, cross-linked derivate of hyaluronan (Tab. 3). Unlike the sodium hyaluronate used in the comparison product INNORYOS 2.2%, Hylan G-F 20 is not of bacterial but of animal origin (rooster comb) [25].
The treatment procedure is equivalent to the test product above
|
|
INNORYOS 2.2% |
Synvisc |
|
Sodium hyaluronate |
22.000 |
8.000 (hylan G-F 20) |
|
Niacinamide |
15.000 |
- |
|
Sodium Chloride |
6.000 |
8.500 |
|
Disodium hydrogen phosphate 2 H2O |
0.563 |
0.160 |
|
Sodium dihydrogen phosphate 2 H2O |
0.045 |
0.040 |
|
Injection water |
in 1 ml |
in 1 ml |
Table 3: Composition of INNORYOS 2.2% and Synvisc
Outcomes
Primary Study Outcome:
- Changes in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score from baseline (visit 1) to 26 week-follow-up (FU) (Visit 5)
Secondary Study Outcomes
- Changes in WOMAC category “pain” from baseline to 26 week- follow-up (FU)
- Changes in WOMAC category “stiffness” from baseline to 26 week-follow-up (FU)
- Changes in WOMAC category “function” from baseline to 26 week-follow-up (FU)
- Changes in WOMAC category “pain” from baseline to 2 week follow-up (Visit 3)
- Adverse effects during the study period
Explanatory outcomes
- Changes in co-medication during the study period
Assessments
Baseline Assessment
During the initial examination of the patients, anthropometric data were recorded and a clinical examination and radiological assessment of the OA were also carried out. In addition, treatment/ medication, physical activity and diet were asked about as part of the anamnesis.
Western Ontario and McMaster Universities Osteoar- thritis Index (WOMAC)
We applied the “Likert (scale)” version (WOMAK LK3 series) of the WOMAC Index that is a self-administered questionnaire predominately applied in the area of hip and knee OA consisting of 24 items divided into 3 categories. Category A determines “pain” (5 items) during walking, using stairs, in bed, sitting or lying, and while standing upright. Category B focuses on “stiffness” after first awaking and later in the day. Category C determines physical function (17 items) while using stairs, rising from sitting, standing, bending, walking, getting in / out of a car, shopping, putting on / taking off socks, rising from bed, lying in bed, getting in / out of bath, sitting, getting on / off toilet, heavy domestic duties, light domestic duties. In the present project, we consistently focus on knee OA. The surveys were conducted as personal interviews during the visits; outcome assessors read out the WOMAC questions to the participants and made the entries.
Visual Analog Scale (VAS)
Additionally to WOMAC, the visual analog scale (VAS) was used for pain assessment. It is an instrument where a person marks their pain on a scale between "no pain" and "worst pain imaginable". It is widely used in research due to its simplicity and ability to provide continuous data on pain intensity. We used a scale of 0-10 for the VAS.
Adverse Effects
An adverse event was defined as any adverse medical event, unanticipated illness or injury, or any adverse clinical sign, including an abnormal laboratory finding, in subjects, users or other persons in a clinical trial, whether related to the investigational product or not. Severe adverse effects were defined as (a) death or (b) a serious deterioration in health resulting in (b1) life-threatening illness or injury, (b2) permanent impairment of a bodily structure or function, (b3) hospitalization or prolongation of the patient's hospital stay, (b4) need for medical or surgical intervention to prevent (b1) or (b2), (b5) chronic illness.
Co-Medication
Co-medication and in particular pain-modulating medication was recorded during each of the visits in order to determine changes in medication that might affect our study outcomes.
Sample Size Calculation
Due to the focus of the directed primary hypothesis on “non- inferiority of effects”, the sample size was calculated using the one-tailed Student's t-test. An effect size of 0.57, a statistical significance level of 0.05 and the discriminatory power of 0.8 were applied to calculate the sample size of this clinical trial [27]. The calculation resulted in a sample size of 41 participants/group, however anticipating missing values and a loss of follow up of 10%, 90 participants were ultimately included and allocated to two equal groups of 45 participants each.
Statistical Analysis
We applied the Intention to treat (ITT) principle that included all participants randomly assigned to the study arms (INNORYOS 2.2% vs. CG) regardless of their loss to follow-up. Due to the very low number of missing values (n=1), we simply applied the last observation carried forward method for imputation. Normal distribution was checked graphically (gg-plots, residual plots). We applied 1-tailed-tests, and Mann-Whitney test (when applicable); significance was accepted at p <0.05. ANCOVA that adjusted
for baseline differences was applied to determine between group differences (i.e. “effects”) after 26 weeks (primary outcome). Absolute treatment effects were transposed to percentage changes from baseline to 1, 2, 14 and 26-week FU (i.e. visit 2-5). Simple frequency accounts were applied to address secondary outcomes. Differences in distribution of categorical variables (Tab.4) were analyzed by Pearson chi-square tests using two tailed tests.
Results
Baseline characteristics of the two groups were displayed in table 4. In summary, significant differences were observed for Kellgren- Lawrence Grade, use of analgesics (NSAID), knee swelling and stiffness with consistently higher degrees of complaints and corresponding more pronounced use of pharmacologic therapy in the control group.
|
Variable |
INNORYOS 2.2% (n=45) |
Control (n=45) |
p |
|
Women/Men [n] |
|
22/23 |
0.833 |
|
Age in total [years] |
66.6±11.0 |
63.5±10.0 |
0.080 |
|
BMI in total [kg/m2] |
30.6±5.1 |
29.7±6.0 |
0.444 |
|
Physical activity [VAS 1-10] |
|
|
0.595 |
|
Knee OA unilateral / bilateral [n] |
26/19 |
28/17 |
0.667 |
|
Joint space narrowing [n] |
45 |
45 |
----- |
|
Osteophytes [n] |
12 |
7 |
0.197 |
|
Cyst formation [n] |
15 |
17 |
0.660 |
|
Subchondral sclerosis [n] |
26 |
32 |
0.186 |
|
Kellgren Lawrence Grade [n] |
|
|
0.014 |
|
|
18 |
6 |
|
|
Grade II |
14 |
17 |
|
|
Grade III |
13 |
22 |
|
|
Analgetics [n] |
22 |
36 |
0.002 |
|
Sysadoa [n] |
0 |
0 |
----- |
|
Physiotherapy [n] |
0 |
0 |
----- |
|
Injection steroidal agents [n] |
1 |
1 |
1.00 |
|
Injection viscosupplements [n] |
1 |
0 |
0.315 |
|
Knee inspection: pain [n] |
45 |
44 |
0.915 |
|
Knee inspection: swelling [n] |
22 |
42 |
0.001 |
|
Knee inspection: effusion [n] |
10 |
16 |
0.163 |
|
Knee inspection: stiffness [n] |
6 |
20 |
0.001 |
|
Pain when walking [VAS 1-10] |
5.20±2.19 |
5.06±12.28 |
0.778 |
Table 4: Baseline Characteristics of the Study Groups (Number; Mean Value± Standard Deviation, 2-Tailed p-Value)
Lost to Follow-Up, Compliance
None of the 90 participants quit the study or were lost to follow- up. Consequently, all participants (n=45 intervention group, n=45 CG) were analyzed. In one case (CG), one item of the WOMAC stiffness category (”later on the day”) was not completed by the participant and was imputed by the method described above (Fig. 1).
Primary study outcome
Figure 2 displays mean value along with 95% confidence interval for percentage improvements in total WOMAC score of the INNORYOS 2.2% and control group. So far, independently of the follow-up assessment date a significant inferiority INNORYOS 2.2% versus the control treatment with Synvisc 0.8%, 2.0 ml) has not been observed (Fig. 2).
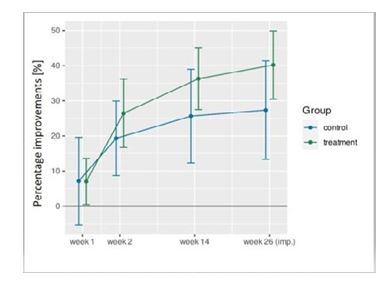
Figure 2: Percentage Improvements of the Total WOMAC-Score in the INNORYOS 2.2% and CG During the Study
Applying ANCOVA that focuses on changes from baseline to 26-week FU and adjusted for baseline differences, no significant inferiority of INNORYOS 2.2% versus the control treatment can be observed for total WOMAC score (p=.290). In detail, WOMAC pain (p=.26) and function (p=.49) scores did not differ significantly between the groups. However, significantly more favorable data after INNORYOS 2.2% were observed for WOMAC stiffness subcategory (p=.009).
In summary, we thus confirmed the primary hypothesis that the average effect of INNORYOS (2.2%, 2.0 ml) on WOMAC total score Index is not inferior to the control group provided with the standard agent (Synvisc 0.8%, 2.0ml).
Secondary Study Outcome
Hypothesis 2a: In summary, the hypotheses of average positive changes of at least 50% in WOMAC “pain”, “stiffness” and “function” subscale after INNORYOS 2.2% injection from baseline to final follow-up assessment have to be rejected for all WOMAC categories. In detail, changes from baseline to 26 week FU average 43.6% for WOMAC “pain”, 25.9% for WOMAC “stiffness” and 37% for WOMAC “function” score. Although not addressed by the hypotheses, average changes among the control group were consistently tendentially lower for all WOMAC categories (i.e., “pain”: 29.1%; “stiffness”: 0%, function 27.6%).
Hypothesis 2b: With respect to the response rate at final FU (26 weeks), we observed positive treatment effects on total WOMAC score in 86.7% of the participants of the INNORYOS 2.2% group. Thus, hypothesis 2b can be confirmed.
Hypothesis 2c: Response rate for the first two weeks (visit 3) after initial INNORYOS 2.2% injection averaged 77.8% and thus slightly exceeded the 75% threshold of positive response rate required by hypothesis 2c.
Pain was rated additionally to WOMAC questionnaire by VAS during walking. Pain reduced to a similar extent in both groups. From baseline to 26 week FU pain dropped from VAS level of 5.2 to 3.4 in the INNORYOS 2.2% group, and from 5.1 to 3.5 in the control group.
Adverse Effects During the Study Period
No adverse effects were observed or reported by the participants.
Confounding Effects
As determined by personal interviews and recorded in the questionnaires, no changes of co-medication with impact on the study outcomes addressed here were observed.
Discussion
This investigation sought to establish the significant non-inferiority of the test compound INNORYOS 2.2% in contrast to the standard agent Synvisc for managing knee OA in individuals aged 40 to 85 years. Synvisc 0.8%, 2.0 ml is one of the most commonly used hyaluronan preparations. It is a high-quality cross-linked HA product whose positive effects have been proven in several studies [20-26]. A systematic review has shown that cross-linked HA has a significantly greater therapeutic effect (p=.003) compared to non-cross-linked HA [28]. The cross-links improve the physical properties of HA by increasing the molecular size, slowing down enzymatic degradation and increasing the retention time in the
joint [29].
However, INNORYOS 2.2% is a high molecular weight HA to which properties similar to those of cross-linked HA are attributed. We assume that INNORYOS 2.2% has the same performance as Synvisc 0.8%, 2.0 ml - unlike non-cross-linked low molecular weight HA [4]. Thus, we formulated the primary hypothesis of non-inferiority with respect to changes in total WOMAC score. The primary hypothesis could be accepted, as it was demonstrated that the average performance, provided with INNORYOS 2.2%, incorporating 1.5% niacinamide, was not inferior to the average performance within the comparison group treated with Synvisc 0.8%, 2.0 ml. With respect to the secondary hypothesis, INNORYOS 2.2% did not achieve the desired treatment outcome of a 50% improvement on the WOMAC “pain”, “stiffness” and “function” subscales at the final follow-up visit at week 26 compared to baseline. The 50% improvement benchmark was predicated on a meta-analysis indicating that "HA improves pain by approximately 40-50% compared with baseline levels" [6]. It is worth mentioning that INNORYOS 2.2% tendentially outperformed the comparative product in all categories. However, statistical significance was only observed for the subscale “stiffness”.
Beside the performance, the tolerability and safety of the test compound were examined through the monitoring of potential adverse events, none of which occurred. INNORYOS 2.2% consists of hyaluronic acid derived from bacterial fermentation. It therefore does not contain any animal material. Synvisc is a cross- linked HA derived from rooster comb. It is reported that patients using biological fermentation-derived HA had fewer cases of acute flare-ups at the injection site than those using avian-derived HA products [30]. However, on the basis of our study data no comparative safety conclusions can be drawn, as no adverse events occurred in either group.
In various contemporary meta-analyses, research emphasis is increasingly gravitating towards alternative conservative treatment modalities such as platelet-rich-plasma (PRP), bone marrow aspirate concentrate, and others [9, 31-33]. These studies indicate a growing superiority of these therapeutic agents, albeit confirming the efficacy of HA as well. Several meta-analyses have been published examining the efficacy of PRP compared to HA. In summary, the studies came to the conclusion that PRP is superior to HA in terms of the total WOMAC score over a study period of one year [34-36]. When looking at a subgroup analysis with high molecular weight and cross-linked HA, no statistically significant difference was found between the use of PRP and this type of HA [35]. In contrast to low molecular weight, non-cross-linked HA, PRP does not appear to be superior compared to high molecular weight and cross-linked HA.
INNORYOS 2.2% is a high molecular weight HA which contains 1.5% of niacinamide, an amide of nicotinic acid, which possesses anti-inflammatory properties and may positively influence degenerative processes in arthritic joints [12]. Thus, INNORYOS 2.2% represents a potent and safe option which can be used in isolation or possibly in combination with other new procedures. Recent investigations suggest that a combination of platelet-rich plasma and HA can exert a particularly favorable influence on degenerative processes in the knee joint [37, 38]. Consequently, the significance of HA therapy is unlikely to wane in the future. Interestingly, a meta-analysis showed that the combination of PRP and HA injections was safer than isolated PRP injections when assessing the incidence of adverse events, which makes the combination an interesting treatment option [39]. It will remain advantageous to formulate the most efficacious HA preparation to afford patients the optimal treatment, be it through monotherapy or combination therapy.
While this study illustrates the non-inferiority of INNORYOS 2.2% compared to Synvisc, Porcello et al. evaluated several HA products, including those aforementioned, with respect to rheological, lubricative, adhesive, and stability attributes [40]. The results showed that INNORYOS 2.2% “presented the best overall functional behavior in the retained experimental settings (high adhesivity and lubricity and substantial resistance to oxidative degradation)” thus surpassing the comparator product Synvisc [40].
Both hyaluronic acid preparations are accessible as a triple application, the customary method in Germany. However, the single application appears to be no less effective than this regimen [41]. As the efficacy of HA injections for OA in various joints, such as the hip or shoulder is currently under investigation with promising outcomes, it can be inferred that INNORYOS 2.2% has an effect on other joints [42-46, 47, 48].
It must be mentioned as a strength that in fact all participants completed the treatment. A key factor in this was certainly the free provision of treatment to a collective with high levels of suffering. In addition, the rapid onset of a positive effect after the first injection motivated many participants to complete the treatment.
Some limitations of our trial should be noted. Of importance, baseline values for Kellgren-Lawrence score differ significant between the groups with higher levels of advanced KL-grade in the CG. However, the prevalence of the single different radiological OA characteristics recorded in the questionnaire (joint space narrowing, osteophytes, cyst formation, subchondral sclerosis) did not differ significantly between the groups. There is some evidence that a higher degree of irreversible changes observed at higher KL-levels might conflict with the treatment effects in the control group [49, 50]. In parallel, significant differences on baseline use of analgesics (Tab. 4) with significantly higher use in the CG might confound our finding. However, considering that no within or between group changes of medication intake were recorded during the study period, we feel that the impact of differences remains negligible.
Conclusion
This study showed that INNORYOS 2.2%, a HA preparation containing niacinamide, is non-inferior to a high molecular weight, cross-linked HA in the treatment of knee OA. While both products exhibited positive effects on pain, mobility, and joint stiffness, the efficacy across all domains was tendentially higher in the group receiving INNORYOS 2.2%, with significant results for “stiffness”. To more precisely address the anti-inflammatory effects presumably induced by niacinamide in the knee, future investigations should record dedicated inflammatory markers. Additionally, a larger cohort and statistical tests for the significant superiority of INNORYOS 2.2% over other HA preparations are warranted.
Funding
This work was supported by ALBOMED, Germany.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgments:
All authors significantly contributed to the study and the manuscript.
The study was financially supported by ALBOMED GmbH, Schwarzenbruck, Germany. ALBOMED GmbH provided the necessary funding for the study and supplied the required preparations to support the research.
The authors declare that there are no further financial dependencies or affiliations with ALBOMED GmbH beyond the support noted above. The authors confirm that there are no additional conflicts of interest, financial or otherwise, related to this publication.
All authors affirm that their contributions to this publication were conducted independently of any influence from ALBOMED GmbH. The design, execution, analysis, and reporting of the study were performed objectively and without external interference from the funder.
Study data was presented as a poster at the DGOU congress in Berlin on October 24, 2024.
References
1. Clynes, M. A., Jameson, K. A., Edwards, M. H., Cooper, C., & Dennison, E. M. (2019). Impact of osteoarthritis on activities of daily living: does joint site matter?. Aging clinical and experimental research, 31, 1049-1056.
2. Vos, T., Lim, S. S., Abbafati, C., Abbas, K. M., Abbasi, M., Abbasifard, M., ... & Bhutta, Z. A. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The lancet, 396(10258), 1204-1222.
3. Long, H., Liu, Q., Yin, H., Wang, K., Diao, N., Zhang, Y., ... & Guo, A. (2022). Prevalence trends of siteâ?Â├é┬Éspecific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis & Rheumatology, 74(7), 1172-1183.
4. Lin, H. S., Watts, J. N., Peel, N. M., & Hubbard, R. E. (2016). Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC geriatrics, 16, 1-12.
5. Zhang, W., Moskowitz, R. W., Nuki, G., Abramson, S., Altman, R. D., Arden, N., ... & Tugwell, P. (2008). OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis and cartilage, 16(2), 137-162.
6. Colen, S., Van Den Bekerom, M. P., Mulier, M., & Haverkamp, D. (2012). Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs, 26, 257-268.
7. He, W. W., Kuang, M. J., Zhao, J., Sun, L., Lu, B., Wang, Y., ... & Ma, X. L. (2017). Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. International Journal of Surgery, 39, 95-103.
8. Petrella, R. J., & Petrella, M. (2006). A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. The Journal of rheumatology, 33(5), 951-956.
9. Singh, Harsh, Derrick M. Knapik, Evan M. Polce, Carlo K. Eikani, Amanda H. Bjornstad, Safa Gursoy, Allison K. Perry et al. "Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network meta-analysis." The American journal of sports medicine 50, no. 11 (2022): 3140-3148.
10. Wang, C. T., Lin, J., Chang, C. J., Lin, Y. T., & Hou, S. M. (2004). Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: a meta-analysis of randomized controlled trials. JBJS, 86(3), 538-545.
11. Webb, D., & Naidoo, P. (2018). Viscosupplementation for knee osteoarthritis: a focus on Hylan GF 20. Orthopedic research and reviews, 73-81.
12. Bains, P., Kaur, M., Kaur, J., & Sharma, S. (2018). Nicotinamide: Mechanism of action and indications in dermatology. Indian journal of dermatology, venereology and leprology, 84, 234.
13. Jonas, W. B., Rapoza, C. P., & Blair, W. F. (1996). The effect of niacinamide on osteoarthritis: a pilot study. Inflammation Research, 45, 330-334.
14. McCarty, M. F., & Russell, A. L. (1999). Niacinamide therapy for osteoarthritis–does it inhibit nitric oxide synthase induction by interleukin-1 in chondrocytes?. Medical hypotheses, 53(4), 350-360.
15. World Medical Association. (2001). Ethical principles for medical research involving human subjects. European journal of emergency medicine: oficial journal of the European Society for Emergency Medicine, 8(3), 221-223.
16. Kellgren, J. H., & Lawrence, J. S. (1957). Radiological assessment of osteo-arthrosis. Ann Rheum Dis, 16(4), 494- 502.
17. Miller, L. E., & Block, J. E. (2013). US-approved intra- articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders, 6, CMAMD-S12743.
18. Richette, P., Chevalier, X., Ea, H. K., Eymard, F., Henrotin, Y., Ornetti, P., ... & Marty, M. (2015). Hyaluronan for knee osteoarthritis: an updated meta-analysis of trials with low risk of bias. RMD open, 1(1), e000071.
19. Bellamy, N., Campbell, J., Welch, V., Gee, T. L., Bourne, R., & Wells, G. A. (2006). Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane database of systematic reviews, (2).
20. Tammachote, N., Kanitnate, S., Yakumpor, T., & Panichkul, P. (2016). Intra-articular, single-shot hylan GF 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. JBJS, 98(11), 885-892.
21. Zhao, H., Liu, H., Liang, X., Li, Y., Wang, J., & Liu, C. (2016). Hylan GF 20 versus low molecular weight hyaluronic acids for knee osteoarthritis: a meta-analysis. BioDrugs, 30, 387-396.
22. Maheu, E., Avouac, B., Dreiser, R. L., & Bardin, T. (2019). A single intra-articular injection of 2.0% non-chemically modified sodium hyaluronate vs 0.8% hylan GF 20 in the treatment of symptomatic knee osteoarthritis: A 6-month, multicenter, randomized, controlled non-inferiority trial. PloS one, 14(12), e0226007.
23. Cortet, B., Lombion, S., Naissant, B., Vidovic, E., & Bruyère, O. (2021). Non-inferiority of a single injection of sodium hyaluronate plus sorbitol to Hylan G-F20: a 6-month randomized controlled trial. Advances in Therapy, 38, 2271- 2283.
24. Bettonville, M., Léon, M., Margaux, J., Urbin-Choffray, D., Theunissen, E., Besse-Hammer, T., ... & Kaux, J. F. (2021). Safety and efficacy of a single intra-articular injection of a novel enhanced protein solution (JTA-004) compared to hylan GF 20 in symptomatic knee osteoarthritis: a randomized, double-blind, controlled phase II/III study. BMC musculoskeletal disorders, 22, 1-15.
25. Ke, Y., Jiang, W., Xu, Y., Chen, Y., Zhang, Q., Xue, Q., ... & Zhu, Z. (2021). Efficacy and safety of a single intra-articular injection of 6 ml Hylan GF 20 compared to placebo in Chinese patients with symptomatic knee osteoarthritis: C-SOUND study, a 26-week multicenter double-blind randomized placebo-controlled trial in China. BMC Musculoskeletal Disorders, 22(1), 428.
26. De Lucia, O., Jerosch, J., Yoon, S., Sayre, T., Ngai, W., & Filippou, G. (2021). One-year efficacy and safety of single or one to three weekly injections of hylan GF 20 for knee osteoarthritis: a systematic literature review and meta- analysis. Clinical Rheumatology, 40(6), 2133-2142.
27. Hummer, C. D., Angst, F., Ngai, W., Whittington, C., Yoon, S. S., Duarte, L., ... & Schemitsch, E. (2020). High molecular weight Intraarticular hyaluronic acid for the treatment of knee osteoarthritis: a network meta-analysis. BMC Musculoskeletal Disorders, 21, 1-10.
28. Jevsevar, D., Donnelly, P., Brown, G. A., & Cummins, D. S. (2015). Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. JBJS, 97(24), 2047-2060.
29. Lindqvist, U., Tolmachev, V., Kairemo, K., Åström, G., Jonsson, E., & Lundqvist, H. (2002). Elimination of stabilised hyaluronan from the knee joint in healthy men. Clinical pharmacokinetics, 41, 603-613.
30. Altman, R. D., Bedi, A., Karlsson, J., Sancheti, P., & Schemitsch, E. (2016). Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. The American journal of sports medicine, 44(8), 2158-2165.
31. Anil, U., Markus, D. H., Hurley, E. T., Manjunath, A. K.,
Alaia, M. J., Campbell, K. A., ... & Strauss, E. J. (2021). The efficacy of intra-articular injections in the treatment of knee osteoarthritis: A network meta-analysis of randomized controlled trials. The Knee, 32, 173-182.
32. Belk, J. W., Lim, J. J., Keeter, C., McCulloch, P. C., Houck, D. A., McCarty, E. C., ... & Kraeutler, M. J. (2023). Patients with knee osteoarthritis who receive platelet-rich plasma or bone marrow aspirate concentrate injections have better outcomes than patients who receive hyaluronic acid: systematic review and meta-analysis. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 39(7), 1714-1734.
33. Kim, J. H., Park, Y. B., & Ha, C. W. (2023). Are leukocyte-poor or multiple injections of platelet-rich plasma more effective than hyaluronic acid for knee osteoarthritis? A systematic review and meta-analysis of randomized controlled trials. Archives of Orthopaedic and Trauma Surgery, 143(7), 3879- 3897.
34. Tan, J., Chen, H., Zhao, L., & Huang, W. (2021). Platelet- rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis of 26 randomized controlled trials. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 37(1), 309-325.
35. Tang, J. Z., Nie, M. J., Zhao, J. Z., Zhang, G. C., Zhang, Q., & Wang, B. (2020). Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Journal of orthopaedic surgery and research, 15, 1-15.
36. Belk, J. W., Kraeutler, M. J., Houck, D. A., Goodrich, J. A., Dragoo, J. L., & McCarty, E. C. (2021). Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. The American journal of sports medicine, 49(1), 249-260.
37. Zhao, J., Huang, H., Liang, G., Zeng, L. F., Yang, W., & Liu,
J. (2020). Effects and safety of the combination of platelet- rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis. BMC musculoskeletal disorders, 21, 1-12.
38. Karasavvidis, T., Totlis, T., Gilat, R., & Cole, B. J. (2021). Platelet-rich plasma combined with hyaluronic acid improves pain and function compared with hyaluronic acid alone in knee osteoarthritis: a systematic review and meta-analysis. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 37(4), 1277-1287.
39. Zhang, Q., Liu, T., Gu, Y., Gao, Y., & Ni, J. (2022). Efficacy and safety of platelet-rich plasma combined with hyaluronic acid versus platelet-rich plasma alone for knee osteoarthritis: a systematic review and meta-analysis. Journal of orthopaedic surgery and research, 17(1), 499.
40. Porcello, A., Hadjab, F., Ajouaou, M., Philippe, V., Martin, R., Abdel-Sayed, P., ... & Laurent, A. (2023). Ex vivo functional benchmarking of hyaluronan-based osteoarthritis viscosupplement products: Comprehensive assessment of rheological, lubricative, adhesive, and stability attributes. Gels, 9(10), 808.
41. Suppan, V. K. L., Tew, M. M., Wong, B. C., Chan, H. K.,
Chew, Y. W., Tan, C. S., ... & Sadashiva Rao, A. (2020). One-year follow-up of efficacy and cost of repeated doses versus single larger dose of intra-articular hyaluronic acid for knee osteoarthritis. Journal of Orthopaedic Surgery, 28(1), 2309499019895029.
42. Belk, J. W., Houck, D. A., Littlefield, C. P., Kraeutler, M. J., Potyk, A. G., Mei-Dan, O., ... & McCarty, E. C. (2022). Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of level I and II randomized controlled trials. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 38(6), 2035-2046.
43. Brander, V., Skrepnik, N., Petrella, R. J., Jiang, G. L., Accomando, B., & Vardanyan, A. (2019). Evaluating the use of intra-articular injections as a treatment for painful hip osteoarthritis: a randomized, double-blind, multicenter, parallel-group study comparing a single 6-mL injection of hylan GF 20 with saline. Osteoarthritis and cartilage, 27(1), 59-70.
44. Nouri, F., Babaee, M., Peydayesh, P., Esmaily, H., & Raeissadat, S. A. (2022). Comparison between the effects of ultrasound guided intra-articular injections of platelet-rich
plasma (PRP), high molecular weight hyaluronic acid, and their combination in hip osteoarthritis: a randomized clinical trial. BMC Musculoskeletal Disorders, 23(1), 856.
45. Sambe, H. G., Yasir, M., Man, R. K., Gogikar, A., Nanda, A., Janga, L. S. N., & Hamid, P. (2023). Comparing Intra- articular Platelet-Rich Plasma With Hyaluronic Acid for the Treatment of Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Cureus, 15(10).
46. Villanova-López, M. M., Núñez-Núñez, M., Fernández-Prieto, D., González-López, C., García-Donaire, J., Pérez-Pérez, A.,... & Ballester-Alfaro, J. J. (2020). Randomized, double-blind, controlled trial, phase III, to evaluate the use of platelet-rich plasma versus hyaluronic acid in hip coxarthrosis. Revista Española de Cirugía Ortopédica y Traumatología (English Edition), 64(2), 134-142.
47. Familiari, F., Ammendolia, A., Rupp, M. C., Russo, R., Pujia, A., Montalcini, T., ... & de Sire, A. (2023). Efficacy of intraâ?Â├é┬Éarticular injections of hyaluronic acid in patients with glenohumeral joint osteoarthritis: A systematic review and metaâ?Â├é┬Éanalysis. Journal of Orthopaedic Research®, 41(11), 2345-2358.
48. Zhang, B., Thayaparan, A., Horner, N., Bedi, A., Alolabi, B., & Khan, M. (2019). Outcomes of hyaluronic acid injections for glenohumeral osteoarthritis: a systematic review and meta-analysis. Journal of Shoulder and Elbow Surgery, 28(3), 596-606.
49. Toh, E. M., Prasad, P. S., & Teanby, D. (2002). Correlating the efficacy of knee viscosupplementation with osteoarthritic changes on roentgenological examination. The Knee, 9(4), 321-330.
50. Altman, R. D., Farrokhyar, F., Fierlinger, A., Niazi, F., & Rosen, J. (2016). Analysis for prognostic factors from a database for the intra-articular hyaluronic acid (Euflexxa) treatment for osteoarthritis of the knee. Cartilage, 7(3), 229- 237



