Research Article - (2025) Volume 4, Issue 2
Dosimetry of Medical Linear and Its Use in The Treatment Planning System
2Alma Mater Europae Campus College “REZONANCA”, Prishtina, Albania
3University Clinical Center of Kosovo, Prishtina, Albania
4Faculty of Mathematical and Natural Science, Department of Physics, University of Prishtina “Hasan P, Albania
Received Date: May 24, 2025 / Accepted Date: Apr 28, 2025 / Published Date: May 05, 2025
Copyright: ©�?©2025 Blerim Rrakaqi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Telhaj, E., Rrakaqi, B., Xhafa, B., Kaciu, Y., Elezaj, N. (2025). Dosimetry of Medical Linear and Its Use in The Treatment Planning System. J Surg Care 4(2), 01-05.
Abstract
Two medical linear accelerators Elekta Synergy Platform are in use on Hygeia Hospital Tirana, for radiotherapy purposes. The high voltage of linear accelerator is between 6 and 18 MV. The dosimetry of the X-rays beam of two accelerators is performed in regular basis by ionization chambers with small volumes. The data of the dosimetric measurements enters into database of XIO treatment planning system (TPS) and along computed tomography (CT) data, serve as initial data for TPS preparing, aiming the irradiation process of the target volume with high precision using one or some radiation fields. Based in our measurements 100 monitor units (MU) gave the dose of 1,009 Gy for high voltage 6 MV and 1,004 Gy for high voltage of 18 MV with uncertainty of 1%.
Keywords
Linear Accelerator, High Energy X-ray Beam, Ionization Chamber, Radiotherapy, Radiation Dosimetry, Monitor Unit
Introduction
Cancer treatment in the last decades is based in the combination of the following three methods: surgery, chemotherapy and radiotherapy. Radiotherapy nowadays along with cobalt machines is using successfully high energy X-rays beam produced by medical linear accelerator of 6 to 20 MV high voltages. Determination of the patient absorbed dose for the mentioned high energy X-ray is performed through its measurements for different depth in water, which ought to meet the special requirements of the International Code of Practice [1]. For this purpose, a series of measurements are performed in standard water depth of 10 cm, which ought to differ less than 2% of the reference dose of 1 Gy. Based in those measurements, the calibration of the device was carried out in terms of monitor unit (MU), which is equal to 1cGy [2]. The measurements data for the X-rays of the two mentioned high voltage devices are used as input database for the TPS XIO, which is turned in a reference system for modeling the high energy X-ray beam of the patients. The beam modeling and the dose calculation of the patients is performed always based in the dosimetric measurements referring to the absorbed dose in 10 cm water depth.
Materials and Methods
Absorbed Dose Measurement
Determination of the patient absorbed dose is based in the measurements of the dose in water, as result of the equality values of the soft tissue and water electronic density. The methodology of the measurements is based on the Bragg-Gray theory, which was improved latter with the following assumptions:
• the small volume of ionization chamber did not change energetic spectrum of the secondary electrons
• high energy photons produce not significant quantity of the secondary electrons inside the ionization chamber
• high energy photon flux is constant in air and its surroundings.
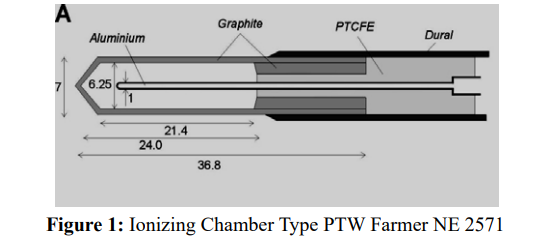
For the measurements of the absorbed dose are used ionization chambers with small volumes. We used Farmer ionization chamber 30013 (PTW) of 0,6 cm3 volume. The electric charge which is formed inside the ionization chamber (400 V) is measured by PTW UNIDOS electrometer, which is calibrated in the terms of the absorbed dose in water ND,w (Figure. 1).
Based in the protocol for clinical reference dosimetry [3], the uncertainty of the calibrated instrument in the SSDL ought to be less than 0,5 %. Calibration factor of the ionization chamber needed to correct for the nominal energy of X-ray beam. This factor is given based in the quality index (QI) of the used beam. The absorbed dose in water for a given QI beam is determined by the formula as follow:
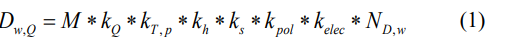
where M is the electric charge collected by electrometer. Corrective factors are respectively for beam quality index, environmental conditions (pressure and temperature), humidity, ion recombination, polarity correction and electrometer calibration.
The last factor (ND,,w) represents the calibration coefficient for the absorbed dose in water by high energy X-ray beam of given QI. The international code (1) recommends that the determination of the quotient for the absorbed dose measurement in the water phantom depth of 10 cm and 20 cm (TPR20 ),and the quotient ofthe ionization in depth of 10 cm and 20 cm ( J10 ) to perform with field size 10x10 cm2. The mentioned protocol confirms that exist the following relation between the dose and ionization quotiens:
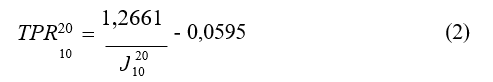
The general uncertainty of the absorbed dose, including all clinical uncertainty, should be less than 1,5%, which is spread between the following factors: long-term stability of used dosimeter 0,3%, reference term stability 0,4%, relative reading of dosimeters 0,6%, correction of the influential units 0,4% and correction of the quality beam 1,0%. The further reduction of the uncertainty is based in the reduction of the mentioned factors for uncertainty [4].
The measurements for the absorbed dose evaluation are carried out for radiation beams of energy which belong to high voltage of 6 MV and 18 MV. These measurements cover the different depth in water and plastic phantom especially for the quotient of the dose (TPR20 ). During a therapeutic treatment by linear accelerator the absorbed dose is managed in terms of the monitor units, which is measured by two independent ionizing chambers, installed to the head of the device. The main purpose of the dose evaluation is to assess the dose into patient as well to determine the necessary number of MU for every treatment beam.
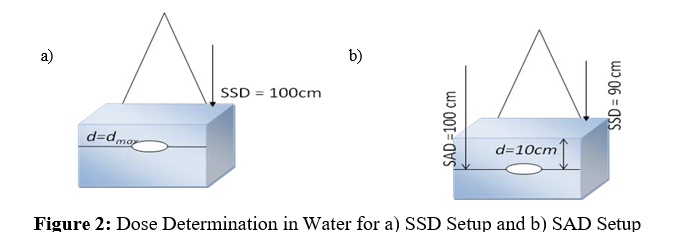
The number of MU for the absorbed dose of 1 Gy (100MU) in 10 cm water depth in the central axis of ionizing chamber is calculated for the field size of 5x5 cm2, 5x7 cm2, 7x7 cm2, 10x10 cm2, 15x15 cm2, 20x20 cm2, 30x30 cm2, 40x40 cm2, 5x30 cm2 and 30x5 cm2. The Output Dose and the Percentage Depth Dose (PDD) for the configuration of the TPS are calculated based in maximum absorbed dose measurements and in absorbed dose in water depth of 10 cm (Figure 2).
Calculation of the Absorbed Dose
In clinical practice the referential methods for calculation of the absorbed dose are based in some algorithms like Monte Carlo, Fast Fourier Transform Convolution, Superposition, Clarkson etc. [5]. In our study the absorbed dose calculation is performed based in the combined referential method Convolution/Superposition. The data for measurement of absorbed dose in water, given on-line, is configured based in the mentioned method for their use later in the TPS, creating the real plan of patient treatment. The combination method Convolution/Superposition is a combination of two methods which are similar and complementary to each other. Both methods use the same physical methodology for dose calculation, but differ in the way of tissues dose deposition. The first method is a fast one, but its uncertainty to the dose calculation especially for non-homogenous tissues is not satisfactory. This method is more suitable for high energy X-ray beam commissioning as well for dose calculation in homogenous tissues. For patients who will be treated in areas with non-homogenous tissues it is recommended to use the combination of the Convolution and Superposition methods.
The first method is represented in Cartesian coordination system related to isocenter, which is constant in the space where the irradiation is applied. The second one is represented in spherical coordination system, for which is allowed to change based in the local change of electronic density in the space irradiation application.
For these algorithms needed to consider the following referential structures and steps:
• Electronic (beam) contamination as function of field value
• Radiation scattering by phantom versus field value
• General radiation scattering versus field value
• Correction factor of filters
The mentioned data are necessary for the determination of the monitor units MU, which is directly related to the patient dose.
The MU required to deliver the prescribe dose, for fixed SSD
treatment, is calculated based in the following relation:

where:
D is the delivery dose (in cGy);
OF is the output factor (standard field size);
PDD is the percent depth dose of the point in question;
WF is the wedge factor (the presence of any beam modifying devices); and
CF is the calibration factor (only important if 1MU is not equal to
1cGy under reference conditions).
In fact, the method of dose deposition is based in an adoption of the analytical method of dose calculation “collapsed cone convolution”. [6].
The final dose calculation for both methods is performed in Cartesian coordination system. From the other side calculated dose for spherical coordination system is interpolated for each point which will serve for irradiation process accuracy (Figure 3).
Figure 3: Spherical Appearance of Calculation and dose Deposition in a Specific Volume
Results and Discussions
The dose calculation relation for a reference depth and for a reference beam is given as follow:
where MQ is the product of electric charge with different corrective factors. Experimental positioning of the absorbed dose measuring system under reference terms is given in advance. Being in such reference terms and using dosimetric system (ionizing chamber, electrometer and programs) the absorbed dose measurement is performed for a water phantom and for high energy X-ray beam of 6 MV and 18 MV high voltage, generated by linear accelerator,
configured and collimated by the system represented in Figure 4
Dw,Q = MQ * ND, w (4)

The measurements are carried out in the distance SSD = 100 cm and in the depth of 10 cm. Central axis of Farmer ionization chamber 0,6 cm3 is positioned in the reference depth for all measured beam energies of 6 MV and 18 MV with field size 10x10 cm2 (4). In such conditions the absorbed dose in water for maximal depth and for standard conditions of temperature, pressure and humidity ought to be 100 cGy for 100 MU with uncertainty of 2% (Figure 5).
Figure 5: PDD Curves for Energies 6 MV and 18 MV at dmax
The measurements of the PPD are performed in the maximal depth. Their normalization is carried out for reference depth of 10 cm, considering that the PPD curves are sum of the photon dose and electron contaminated dose. The curves normalization process may be performed in maximal depth as well in reference depth (Figure 6 and Figure 7.
The most accurate portion of any Convolution or Superposition calculated PDD is that portion beyond the electron contamination range. The calculated PDD curve, which is the sum of the photon dose and electron contamination dose, can be normalized at depth of 10 cm or at dmax. If one normalizes this PDD to be equal to 100% at dmax, then the calculated doses agree with measurements at dmax, but are in error at all other depths by the 1% error in the electron contamination
(the 1% error in he electron contamination manifests itself at depth). However, one normalizes both curves to equal 100% at a depth of 100 cm the two curves agree everywhere except in the electron contamination region (1% error in the electron contamination is confined to the electron contamination region). The selection of the reference depth at a depth beyond the maximum depth of the electron contamination is because accuracy at dmax is typically less important than accuracy at depth beyond dmax (i.e. clinically relevant depths). The depth of 10 cm is used as reference depth for all photons beam of energy up to 25 MV as result of absorbed dose determination for all mentioned photon beams. The absolute dosimetry for linear accelerator calibration is performed through positioning of the CAX of ionization chamber in 10 cm water depth at 100 cm distance (SSD = 100 cm) to achieve the dose of 1 Gy = 100 MU based in the following relation [7]:
where all factors have the same definition as in formula (1). The values of kpol and ks are measured by PTW Farmer dosimeter a
|
PTW Farmer Dosimeter |
kpol |
ks |
|
6 MV |
1,002 |
1,002 |
|
18 MV |
1,002 |
1,003 |
Dose determination for the depth of 10 cm and correction of the PDD inversion is carried out based in the reference depth dose. The values of the measurements are derived by the commissioning process, positioning the ionization chamber in the effective point of measurement.
The results of the measurement are represented in the following table:
|
Energy / dmax |
Reference dose in 10 cm |
Correction 1/PDD |
|
6 MV / 1,5 cm |
67,3% |
1,486 |
|
18 MV / 2,8 cm |
77,4% |
1,292 |
In the treatment planning system (TPS) XiO the maximal depth of the contaminated electron is accounted considering the energetic model by the on-line measurements which are corrected by a coefficient of 0,4 resulting by the analytical method of “collapsed cone convolution”.
Conclusion
Based in given results, which are referring to the evaluation of the absorbed dose to reference depth dref =10 cm in water, according to internationals protocols, in base of a calibrated dosimetry system, we conclude that [1,3].
1. Convolution/Superposition method can serve as an important tool for dose evaluation, because the reference depth might be selected in such a way that it is beyond the maximum depth of electron contamination.
2. The selection of a reference depth, beyond the maximum depth of the electron contamination, is always possible because the accuracy at dmax is typically less important than accuracy at depths beyond dmax (clinically relevant depth).
3. The calculated PPD curve, which is the sum of the photon dose and electron contamination dose can be normalize at depth of 10 or dmax.
4. The absolute dosimetry for linear accelerator calibration is performed through positioning of the ionization chamber center in 10 cm water depth in the distance of 100 cm (SSD = 100 cm) to achieve the dose of 1 Gy = 100 MU
5. Dose determination for the depth of 10 cm and correction ofPDD inversion is carried out based in the reference depth dose. The values of the measurements are derived by the commissioning process, positioning the ionization chamber in the effective point of measurement.
References
1. Musolino, S. V. (2001). Absorbed dose determination in external beam radiotherapy: an international code of practice for dosimetry based on standards of absorbed dose to water; technical reports series No. 398.
2. Dutreix, A. (1997). Monitor unit calculation for high energy photon beams, Physics for Clinical Radiotherapy, ESTRO B-3.
3. Hanson, W. F., Cho, S., Lowenstein, J., & Balter, P. (1999). AAPM Task Group 51. Med Phys, 26, 1847-70.
4. International Commission on Radiation Units and Measurements. 2000 Dosimetry of High-Energy Photon Beams Based on Standards of Absorbed Dose to Water, ICRU Report 64, Bethesda, MD. ICRU.
5. Knöös, T., Wieslander, E., Cozzi, L., Brink, C., Fogliata, A., Albers, D., ... & Lassen, S. (2006). Comparison of dose calculation algorithms for treatment planning in external photon beam therapy for clinical situations. Physics in Medicine & Biology, 51(22), 5785.
6. Ahnesjö, A. (1989). Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media. Medical physics, 16(4), 577-592.
7. Palmans, H., Nafaa, L., De Jans, J., Gillis, S., Hoornaert, M. T., Martens, C., ... & Vynckier, S. (2002). Absorbed dose to water based dosimetry versus air kerma based dosimetry for high-energy photon beams: an experimental study. Physics in Medicine & Biology, 47(3), 421.