Review Article - (2022) Volume 7, Issue 1
Acute lung injury after pulmonary resection surgery for lung cancer and anesthetic management
2Department of Anesthesiology, Keio University School of Medicine, Japan
Received Date: Dec 27, 2021 / Accepted Date: Jan 02, 2022 / Published Date: Jan 13, 2022
Copyright: ©Copyright: ©2022: Takeshi Suzuki, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Citation: Takeshi Suzuki, Kenta Wakaizumi, Jungo Kato, Takashige Yamada, Hiroshi Morisaki (2022) Acute lung injury after pulmonary resection surgery for lung cancer and anesthetic management. Journal of Clinical Review & Case Reports 7(1):797-803.
Abstract
Although the outcome of patients undergoing pulmonary resection surgeries for lung cancer has improved, postoperative acute lung injury (ALI) remains the leading cause of death. High oxygen concentration, high peak airway pressure, and hyper-perfusion due to hypoxic pulmonary vasoconstriction play a major role in the development of ALI in the ventilated dependent lung during one-lung ventilation. In the collapsed non-dependent lung, mechanical stress induced by re-expansion, ischemia-reperfusion injury, and direct surgical manipulation are associated with the onset of ALI. These contributing factors to ALI elicit local inflammatory responses, resulting in pulmonary edema that resembles acute respiratory distress syndrome histologically. Some preventive strategies to reduce ALI are recommended during mechanical ventilation, OLV support in particular. A lung protective strategy, including sufficient positive end-expiratory pressure, low tidal volume, and lower inspiratory oxygen concentration, should be adopted to attenuate lung damage. The application of continuous positive airway pressure to the collapsed non-dependent lung has been shown to reduce inflammatory cytokines. Given that excessive fluid administration is associated with the risk of postoperative pulmonary complications, a fluid restrictive strategy should be considered to prevent ALI. Anesthetic management may affect the onset of postoperative ALI. Compared to propofol anesthesia, volatile anesthetics have been shown to attenuate local inflammatory responses in both lungs during thoracic surgeries. Thoracic epidural analgesia may attenuate a local inflammatory response in the lung through the blockade of sympathetic nerve stimulation. Thus, more effective strategies to prevent ALI should be investigated to improve the prognosis of patients undergoing thoracic surgeries
Keywords
Thoracic Surgery, Acute Lung Injury, One-Lung Ventilation, Preventive Strategies, Anesthetic Management
Abbreviations
ALI: Acute Lung Injury, ARDS: Acute Respiratory Distress Syndrome, BALF: Bronchoalveolar Lavage Fluid, CPAP: Continuous Positive Airway Pressure, HPV: Hypoxic Pulmonary Vasoconstriction, IL: Interleukin, NO: Nitric Oxide, OLV: OneLung Ventilation, PEEP: Positive End-Expiratory Pressure, TEA: Thoracic Epidural Analgesia, TNF: Tumor Necrosis Factor.
Introduction
Surgical pulmonary resection is the first-choice therapy for patients with early-stage lung cancer. Due to marvelous advances in medical technology, the survival rate of patients undergoing pulmonary resection surgeries has been improved and the incidence of complications has been declined remarkably [1, 2]. However, postoperative complications such as atelectasis, infection of the surgical site, pneumonia, empyema, sepsis, and respiratory failure requiring mechanical ventilatory support, sometimes occur. Among these complications, postoperative acute lung injury (ALI) remains the leading cause of death after surgical pulmonary resection [3]. Therefore, the attenuation of postoperative ALI is a key to further improve the prognosis of patients with lung cancer. Although many factors, including surgical manipulation, barotrauma to alveoli caused by mechanical ventilation, and onelung ventilation (OLV), have been shown to contribute to the development of postoperative ALI [4], the exact mechanisms and the effective measures for its prevention remain unclarified fully yet. In the last two decades, some excellent review articles elucidated the pathophysiology of ALI after thoracic surgery under OLV, and discussed the effective preventive strategies against postoperative ALI [5-7]. However, some randomized controlled trials to investigate preventive measures for postoperative ALI after thoracic surgery were performed after these reviews were published. Thus, an updated new review article is warranted to clarify the pathophysiological mechanisms and discuss preventive measures regarding ALI for improving the prognosis of patients undergoing pulmonary surgery for lung cancer. In this narrative review including recent randomized clinical trials, current knowledge regarding the pathophysiology and preventive strategies for postoperative ALI after lung cancer surgery is reviewed and discussed. Especially, specific anesthetic management to attenuate the development of ALI was debated, such as choice of anesthetic agents, usage of thoracic epidural analgesia (TEA), detailed mechanical ventilatory support during OLV, fluid management, and steroid therapy. All recent important literatures regarding ALI after thoracic surgery were identified from the PubMed using following terms, thoracic surgery, pulmonary surgery, lung cancer, OLV, ALI, pathophysiology, respiratory complications, anesthetic management, anesthetic agents, TEA, preventive strategy, and improvement of outcome. In this manuscript, “ALI” is referred to as a “primary lung injury” that is caused by surgical and anesthetic procedures during lung cancer surgery.
Incidence and Pathophysiology of ALI after Pulmonary Surgery for Lung Cancer
The morbidity and mortality rates of thoracic surgery for lung cancer have been declined mainly due to advanced surgical techniques, anesthetic management, and postoperative care [2]. However, the incidence of ALI, one of the most severe pulmonary complications, has been reported to range from 2% to 7% [8,9]. Besides, once postoperative ALI developed, the mortality rate goes up to more than 20% [8,10]. The frequency of ALI differs depending on the patient characteristics and the type of surgical procedure. For example, advanced age, diabetes mellitus, smoking habits, alcohol abuse, and chronic obstructive pulmonary disease have been reported as major contributors to the development of ALI after thoracic surgery [11,12]. Furthermore, the rate of postoperative ALI after extensive resections was higher compared to those after lobectomies [4]. Another study has shown that predicted postoperative lung perfusion of <55% compared with the preoperative condition, predicted reduced postoperative FEV1 of < 45% and preoperative chemotherapy or radiotherapy were found to be significant risk factors for developing ALI that presented as pulmonary edema [13].
The histological features of ALI after lung cancer surgery, such as exudative alveolar edema, neutrophil accumulation, and hyaline membrane deposition are basically consistent with so-called acute respiratory distress syndrome (ARDS) [14]. Recent evidence suggests that high inflammatory response plays a pivotal role in the development of ALI after lung cancer resection [3], the same mechanisms as ARDS. Increased proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β are likely to alter microvascular permeability and induce an excessive activation of neutrophils, leading to pulmonary edema. While OLV has been shown to contribute to these histological changes of ALI [6], are the actual mechanisms of lung damage quite different between the ventilated dependent and collapsed non-dependent lungs during OLV?
Deleterious Effects of OLV
OLV is a mandatory anesthetic technique during thoracic surgery to provide a sufficient surgical field. However, OLV has been recognized as a major contributing factor to develop ALI after thoracic surgery [15]. Although an intense inflammatory response is one of the major causes of ALI in both ventilated and collapsed lungs as described previously, each lung is damaged through a different pathophysiologic mechanism [6].
In the ventilated lung, oxygen toxicity due to its high concentration delivered to maintain sufficient oxygenation, lung overdistension due to high peak airway pressure to provide a sufficient tidal volume, and hyper-perfusion due to circulatory distribution from the collapsed lung play major roles in the development of ALI [6, 16]. High concentration oxygen elicits lung injury through oxidative stress induced by the generation of reactive oxygen species. High peak inspiratory pressure has been shown to be associated with ALI in patients undergoing a pneumonectomy [17]. Injuries to the alveolar-capillary membrane caused by high inspiratory pressure result in the release of high concentrations of cytokines, accumulation of neutrophils, and increased alveolarcapillary permeability, leading to further lung injury [18]. Due to deleterious effects of high pressure on the alveolar-capillary membrane, the breakdown of glycocalyx, which maintains the integrity of the alveolar-capillary membrane by regulating oncotic pressure, modulating plasma-endothelial cell interactions, and preventing leukocyte and platelet adhesion to the endothelium [19], contributes to increased alveolar-capillary permeability, capillary leak, and alveolar edema formation [20]. During OLV, blood flow to the collapsed lung is distributed to the ventilated lung due to hypoxic pulmonary vasoconstriction (HPV), which is a compensatory mechanism to improve oxygenation, resulting in an approximately 50% increase in pulmonary blood flow to the ventilated lung [20,21]. This increased blood flow further damages glycocalyx due to high capillary blood pressure, leading to lung injury, as described above.
On the other hand, in the collapsed lung, mechanical stress and strain exposed to the alveolar units during lung expansion from the collapsed state, ischemia-reperfusion injury, and direct surgical manipulation are major contributing factors to ALI during and after OLV [6,16]. Re-expansion of the collapsed lung creates high shear stress to the alveolus, which elicits an overproduction of inflammatory cytokines in the collapsed lung [22]. This increased cytokine production could further exacerbate lung injury, not only in the collapsed lung but also in the ventilated lung. Ischemiareperfusion injury is also associated with the development of ALI. During OLV, the collapsed lung is exposed to hypoperfusion because of HPV, leading to hypoxemic changes. In the rat pneumothorax model, the levels of reactive oxygen species were increased in the collapsed lung [23]. Furthermore, some clinical studies have shown that oxidative stress is induced after OLV during a lobectomy for lung cancer, as reflected by elevated hydrogen peroxide and hydrogen ions in exhaled condensates, and increased myeloperoxidase levels in bronchoalveolar lavage samples [24-26]. The degree of oxidative stress and inflammatory response increases with a longer duration of OLV [24,26]. Ischemiareperfusion injury through increased oxidative stress levels causes molecular and cellular damage in the lung and induces further deterioration of the damaged glycocalyx [27]. Surgical trauma also causes diffuse alveolar damage, including alveolar edema, lung parenchyma hemorrhage, neutrophil accumulation, and enhanced local inflammatory response [6]. The impairment in lymphatic drainage caused by surgical manipulation also contributes to lung injury, particularly pulmonary edema [4,28].
Collectively, a number of factors and substantial mechanisms are associated with the development of ALI in both lungs during OLV for pulmonary resection surgery.
Strategies for Anesthetic Management to Prevent ALI after Pulmonary Resection Surgery for Lung Cancer
Without question, we anesthesiologists should prioritize the prevention of ALI since it is the leading cause of death after pulmonary resection surgery. Some preventive strategies to reduce the incidence of ALI have been recommended in previous reports [1,4,6,13,28].
Ventilatory strategy during OLV
Compared with the collapsed lung, ALI is more prone to occur in the ventilated lung during OLV [16]. As managing patients with ARDS in the intensive care unit, lung-protective ventilation is mandatory to attenuate the damage to the ventilated lung caused by inappropriate ventilatory settings.
An increase in the tidal volume of 1 mL/kg of predicted body weight was found to increase the incidence of ALI in 146 pneumonectomy patients [17], and some randomized trials have demonstrated that high tidal volume during OLV contributes to the development of pulmonary complications [29,30]. Furthermore, recent randomized controlled trials have shown that low tidal volume of 4 ml/kg reduced extravascular lung water during OLV for thoracoscopic surgery, compared with larger tidal volume of 6 to 8 ml/kg [31], and 5 ml/kg low tidal volume ventilation was associated with lower major postoperative complications and shorter hospital stay, compared with tidal volume of 10 ml/kg, in patients undergoing lung cancer surgery [32]. Thus, a restricted tidal volume of between 4 to 5 ml/kg of predicted body weight is recommended to suppress lung strain, which is one of the major factors in lung injury caused by mechanical ventilation, and to reduce postoperative respiratory complications.
Positive end-expiratory pressure (PEEP) should be applied to improve oxygenation and prevent lung injury by restoring endexpiratory lung volume and attenuating atelectrauma in the ventilated lung [6,16]. Compared with zero PEEP, patients with 4 cmH2 O PEEP level during OLV have displayed improved oxygenation and decreased lung injury markers [33]. One PEEP decrement trial found that an average PEEP level of 10 cmH2 O was optimal in patients ventilated with 5-7 mL/kg tidal volume during OLV [34]. While the best PEEP level varies depending on each patient’s respiratory condition, anesthesiologists had better adjust PEEP levels continuously through lung recruitment and PEEP titration to maintain oxygenation and to reduce damage to the ventilated lung. In addition, continuous positive airway pressure (CPAP) to the collapsed lung during OLV has also been shown to reduce the local immune response in patients undergoing esophagectomy [35]. Application of CPAP to the collapsed lung attenuated the increase in inflammatory cytokines, such as TNF-α, IL-1β, IL-8, and IL-10, in bronchoalveolar lavage fluid (BALF) collected from the collapsed lung, compared to the non-CPAP group. Collectively, CPAP applied to the collapsed lung may attenuate the degree of postoperative ALI by reducing the local inflammatory response and improving oxygenation during OLV. However, the best PEEP level applied to the collapsed lung during OLV remain to be elucidated yet.
Delivery of high oxygen concentrations to keep sufficient SpO2 during OLV should be avoided because of damage to the lung through oxidative stress. High FIO2 at the initiation of OLV should be gradually reduced according to the decrease of shunt blood flow elicited by HPV after 20-30 minutes of OLV [36]. Reduction of oxygen concentration can also attenuate absorption atelectasis in the ventilated lung.
Respiratory ratio should be reduced as much as possible to attenuate mechanical stress to the alveoli, leading to the reduction of cytokine release shown in animal models [37,38]. Although extreme hypercapnia above 70 mmHg can elicit elevated intracranial pressure, myocardial dysfunction, cardiovascular instability, and pulmonary hypertension, mild hypercapnia (40-60 mmHg) is well tolerated and may suppress ventilator-induced lung injury [39,40].
Recruitment maneuvers during OLV may be an effective method to improve the distribution of ventilation and oxygenation by opening the area of atelectasis and improving lung compliance since atelectasis and ventilation-perfusion mismatch are common in OLV. Although a study by Park et al. showed that the preemptive alveolar recruitment maneuver improved oxygenation during OLV [41], no randomized trials to date have demonstrated its significant effects to prevent postoperative lung injuries.
In several clinical trials, such lung-protective strategies as discussed above attenuated systematic inflammatory responses and reduced the incidence of ALI after thoracic surgery [42-44]. However, the ventilatory strategy should be individualized depending on the condition of each patient to minimize the risk of lung injury. The clinical randomized trial that investigates the effect of an individualized ventilatory strategy in patients undergoing thoracic surgery with OLV is ongoing now [45]. Anesthesiologists should consider that appropriate management of mechanical ventilation during OLV, which should be adjusted in accordance with each patient’s respiratory condition, can result in less lung damage and improve the prognosis of patients undergoing pulmonary resection surgery for lung cancer.
Fluid management
Administration of excessive fluid during pneumonectomies is associated with a higher risk of postoperative pulmonary complications [46, 47], thereby a perioperative fluid restriction strategy is recommended for lung cancer surgery. The relationship between perioperative extra fluid loading and the risk for ALI has been reported over the last couple of decades. In a study by Partin et al., an intraoperative fluid balance of more than 2000 mL was significantly associated with postoperative pulmonary edema in patients undergoing a pneumonectomy [48]. Licker et al. reported that patients developing ALI received a larger perioperative cumulative fluid balance compared with non-ALI patients (2.6 vs. 2.0 mL/kg/h) [8]. To reduce such adverse effects of excessive fluid loading, the initiation of inotropic/vasopressor is recommended if hypoperfusion exists, despite a >1.5 L positive fluid balance [49]. While one retrospective study involving 1,442 thoracic surgery patients showed that intraoperative restricted fluid administration of 2 to 3 mL/kg/h had no association with the onset of acute kidney injury [50], anesthesiologists should ensure adequate perfusion to vital organs when limiting fluid administration during pulmonary resection surgery. However, it is quite difficult to restrict fluid administration as much as possible to attenuate the onset of ALI while keeping adequate blood flow to vital organs during pulmonary resection surgery.
There is no consensus as to whether blood product transfusion has an impact on ALI and how blood transfusion should be managed during pulmonary resection surgery. Although some studies have identified blood transfusion as a risk factor for postoperative respiratory failure in patients undergoing lung resection [51,52], this issue remains controversial.
Steroids
Since an increased inflammatory response is associated with the occurrence of ALI after lung cancer surgery, administration of steroids to suppress inflammation may be able to minimize the incidence of ALI. In one study, involving 72 patients undergoing a pneumonectomy, 250 mg of methylprednisolone administered before pulmonary artery ligation reduced the incidence of postoperative pulmonary edema compared to the control group (0 vs. 13.5%, p=0.049) [53]. Steroid therapy, however, has been shown to provide no beneficial impact on the prognosis of general ALI/ARDS patients [54], and could delay wound healing and increase the incidence of postoperative infection through immunosuppressive effects, thus, the administration of steroids to prevent ALI after pulmonary resection surgery is not recommended.
Effect of Anesthetic Agents on Postoperative ALI after Lung Cancer Surgery
The choice of anesthetic agents for lung cancer surgery may affect the severity of postoperative ALI. Some previous reports demonstrated that volatile anesthetics, compared to propofol, contributed to the attenuation of the local inflammatory response in both ventilated and collapsed lungs in thoracic surgery with OLV [55-57]. In a study by Schilling et al., in which 30 thoracic surgery patients were enrolled, desflurane anesthesia reduced alveolar granulocytes accumulation, TNF-α, and soluble intercellular adhesion molecule-1 in the BALF collected from the ventilated lung, compared to propofol anaesthesia [57]. In another study, the effects of three anesthetic agents (propofol, sevoflurane, and desflurane) on local alveolar and systemic inflammatory responses were examined in 63 thoracic surgery patients. Both 2 volatile anesthetic agents attenuated the local alveolar inflammatory response, as reflected by the decrease of proinflammatory cytokines in the BALF of the ventilated lung, compared with propofol, without affecting the systemic inflammatory response [55]. De Conno et al. also examined the effect of sevoflurane anesthesia on inflammatory markers in the collapsed lung during OLV in 54 patients undergoing thoracic surgery. The alveolar inflammatory markers in the BALF collected from the collapsed lung, including TNF-α, IL-6, IL-8, and monocyte chemoattractant protein 1, were less elevated in the sevoflurane group than in the propofol group [56].
The exact mechanisms by which volatile anesthetic agents mitigate local inflammatory responses in both ventilated and collapsed lungs remains to be elucidated. However, some possible underlying mechanisms for this immunomodulatory effect can be considered. One experimental study suggested that volatile anesthetics have potent inhibitory effects on neutrophil function [57, 58]. Attenuated activation of neutrophils by volatile anesthetics may contribute to suppressing the inflammatory response, as reflected by reduced alveolar cytokines. Another possible mechanism is the reduction of nitric oxide (NO) production by the interaction with inducible NO synthetase [59], caused by reversible inhibition of voltage-dependent calcium channels, and subsequent intracellular calcium concentration decreases through exposure to volatile anesthetics [60]. Such reduction of NO synthesis is likely to alleviate the degree of lung injury. In addition, suppression of HPV by volatile anesthetics might be associated with reduced ischemiareperfusion injury, leading to attenuation of lung injuries [61]. The vasodilation effect of volatile anesthetics on pulmonary arteries might prevent hypoxic lung injuries in the collapsed lung during OLV and attenuate the consequent ischemic reperfusion injury
Collectively, volatile anesthetics have been shown to suppress local inflammatory responses in both lungs during OLV in some clinical studies, whereas it remains unclear as to whether such beneficial effects help to reduce postoperative ALI and improve the overall outcomes of patients undergoing pulmonary resection surgery for lung cancer.
Potential of TEA to Attenuate ALI
TEA has been applied to control stress responses and perioperative pain in thoracic and abdominal surgeries. The efficient analgesia with TEA can enable thoracic and abdominal surgery patients to cough up enough phlegm and initiate an early rehabilitation, resulting in reduced postoperative pulmonary complications and early hospital discharge. Furthermore, since the activity of sympathetic nervous system influences the immune system [62], the neuroendocrine response blunted by TEA could modulate postoperative immune function. Fares et al. showed that TEA reduced the systemic inflammatory response reflected by reduction of systemic IL-6 and IL-8 in patients undergoing esophagectomy with protective lung ventilation [63]. Another study revealed that TEA under a sufficient depth of general anesthesia attenuated excessive production of systemic inflammatory cytokines (TNF-α, IL-6, and IL-10), compared to general anesthesia alone in 120 patients undergoing colonectomy [64]. Besides, Su et al. reported that TEA, combined with general anesthesia, reduced postoperative inflammatory responses by reducing inflammatory cytokines, and improved postoperative cognitive function in elderly patients undergoing liver cancer surgeries [65].
To our knowledge, however, no study examined the effect of TEA on the development of ALI after thoracic surgery. Our recent clinical study revealed that TEA reduced the IL-6 concentration in the epithelial lining fluid collected from the collapsed lung in patients undergoing pulmonary resection surgery for ling cancer [66]. Considering the pivotal roles of sympathetic nervous system in immune function, it is plausible that TEA could influence lung injuries during and after thoracic surgery by modulating systemic and local inflammatory responses. One review article suggested a possibility that TEA induces a reduction of partial arterial oxygen pressure through an increased pulmonary shunt during OLV [67]. Since this increased pulmonary shunt could be caused by pulmonary arterial vasodilation and reduced HPV, TEA during pulmonary resection surgery might alleviate ischemic-reperfusion injury after OLV due to suppressed HPV, leading to attenuation of lung injury
Conclusion
In this narrative review, the pathophysiology and preventive measures of ALI after pulmonary resection surgery were discussed. While various mechanisms contribute to ALI after pulmonary resection surgery (Figure 1), systemic and local inflammatory responses have been shown to be strongly associated with the onset and degree of ALI. Moreover, deleterious effects on the lungs caused by mechanical ventilation and physiological changes induced by OLV are major factors that contribute to the progression of perioperative lung injury. Protective lung strategies are particularly important to attenuate the damage to the ventilated lung during OLV. The use of volatile anesthetic agents and TEA should be carefully considered as they might attenuate ALI after pulmonary resection surgery. Although a novel therapy, administration of serine protease inhibitor to protect endothelial glycocalyx layer, which plays a key role in vascular permeability, was tried to prevent ALI in a clinical study [68], this therapy is far away from a clinical application yet. Further studies to establish preventive strategies against ALI are warranted to further improve the prognosis of patients undergoing pulmonary resection surgery for lung cancer.
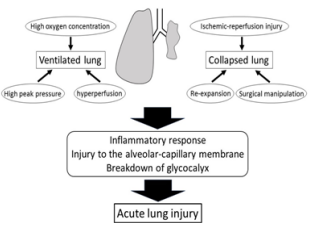
Figure 1: Contributing factors to the onset of acute lung injury after pulmonary resection surgery for lung cancer.
Acknowledgement
None.
Financial Support and Sponsorship
None.
Conflict of Interest Statement
There are no conflicts of interest.
References
1. Eichenbaum KD, Neustein SM (2010) Acute lung injury after thoracic surgery. J Cardiothorac Vasc Anesth 24: 681-690.
2. Slinger PD (2003) Acute lung injury after pulmonary resection: More pieces of the puzzle. Anesth Analg 97: 1555-1557.
3. Alam N, Park BJ, Wilton A, Sehan VE, Bains MS, et al. (2007) Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 84: 1085-1091.
4. Gothard J (2006) Lung injury after thoracic surgery and onelung ventilation. Curr Opin Anaesthesiol 19: 5-10.
5. Kilpatrick B, Slinger P (2010) Lung protective strategies in anaesthesia. Br J Anaesth 105: i108-i116.
6. Lohser J, Slinger P (2015) Lung injury after one-lung ventilation: A review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg 121: 302-318.
7. Senturk M Slinger P, Cohen E (2015) Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Prac Res Clin Anaesthesiol 29: 357-369.
8. Licker M, de Perrot m, Spiliopoulos A, Robert J, Diaper J, et al. (2003) Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 97: 1558-1565.
9. Licker M, Fauconnet P, Villiger Y, Tschopp JM (2009) Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol 22: 61-67.
10. Watanabe S-I, Asamura H, Suzuki K, Tsuchiya R (2004) Recent results of post-operative mortality for surgical
resection in lung cancer. Ann Thor Surg 78: 999-1003.
11. Fernandez-Perez ER, Sprung J, Afessa B, Warner DO, Vachon CM et al. (2009) Intraoperative ventilator settings and acute lung injury after elective surgery: A nested case control study. Thorax 64: 121-127.
12. Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP et al. (2002) Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med 136: 25-36.
13. Parquin F, Marchal M, Mehiri S, Herve P, Lescot B (1996) Post-pneumonectomy pulmonary edema: Analysis and risk factors. Eur J Cardiothorac Surg 10: 929-932.
14. Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342: 1334-1349.
15. Lohser J (2008) Evidence-based management of one-lung ventilation. Anesthesiol Clin 26: 241-272.
16. Bender SP, Anderson EP, Hieronimus RI, Bensimhon A (2018) One-lung ventilation and acute lung injury. Int Anesthesiol Clin 56: 88-106.
17. Jeon K, Yoon JW, Suh GY, Kim J, Kim K, et al. (2009) Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care 37: 14-19.
18. Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369: 2126-2136.
19. Woodcock TE, Woodcock TM (2012) Revised starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br L Anaesth 108: 384-394.
20. Collins SR, Blank RS, Deatherage LS, Dull RO (2013) Special article: the endothelial glycocalyx: emerging concepts in pulmonary edema and acute lung injury. Anesth Analg 117: 664-674.
21. Broccard AF, Hotchkiss JR, Kuwayama N, Olson DA, Jamal S, et al. (1998) Consequences of vascular flow on lung injury induced by mechanical ventilation. Am J Respir Crit Care Med 157: 1935-1942.
22. Tremblay LN, Slutsky AS (2006) Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32: 24-33.
23. Sivrikoz MC, Tuncozgur B, Cekman M, Bakir K, Meram I, et al. (2002) The role tissue reperfusion in the reexpansion injury of the lungs. Eur J Cardiothorac Surg 22: 721-727.
24. Misthos P, Katsaragakis S, Milingos N, Kakaris S, Sepsas E, et al. (2005) Postresectional pulmonary oxidative stress in lung cancer patients. The role of one-lung ventilation. Eur J Cardiothorac Surg 27: 379-382.
25. Cheng YJ, Chan KC, Chien CT, Sun WZ, Lin CJ (2006) Oxidative stress during 1-lung ventilation. J Thorac Cardiovasc Surg 132: 513-518.
26. Garcia-de-la-Asuncion J, Garcia-del-Olmo E, Perez-Griera J, Marti F, Galan G, et al. (2015) Oxidative lung injury correlates with one-lung ventilation time during pulmonary lobectomy: a study of exhaled breath condensate and blood. Eur J Cardiothorac Surg 48: e37-e44.
27. Chappell D, Heindl B, Jacob M, Annecke T, Chen C, et al. (2011) Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 115: 483-491.
28. Bigatello LM, Allain R, Gaissert HA (2004) Acute lung injury after pulmonary resection. Minerva Anestesiol 70: 159-166.
29. Shen Y, Zhong M, Wu W, Wang H, Feng M, et al. (2013) The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: a randomized and controlled study. J Thorac Cardiovasc Surg 146:1267-1273.
30. Yang M, Ahn HJ, Kim K, Kim JA, Yi CA, et al. (2011) Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery? A randomized controlled trial. Chest 139:530-537.
31. Qutub H, El-Tahan MR, Mowafi HA, El Ghoneimy YF, Regal MA, et al. (2014) Effects of tidal volume on extravascular lung water content during one-lung ventilation for video-assisted thoracoscopic surgery. Eur J Anaesthesiol 31:466-473.
32. Marret E, Cinotti R, Berard L, Piriou V, Jobard J, et al. (2018) Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery. Eur J Anaesthesiol 35:727-735.
33. Senturk NM, Dilek A, Camci E, Senturk E, Orhan M, et al. (2005) Effects of positive end-expiratory pressure on ventilatory and oxygenation parameters during pressurecontrolled one-lung ventilation. J Cardiothorac Vas Anesth 19:71-75.
34. Ferrando C, Mugarra A, Gutierrez A, Carbonell JA, Garcia M, et al. (2014) Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg 118: 657-665.
35. Verhage RJ, Boone J, Rijkers GT, Cromheecke GJ, Kroese AC, et al. (2014) Reduced local immune response with continuous positive airway pressure during one-lung ventilation for oesophagectomy. Br J Anaesth 112: 920-928.
36. Ishikawa S (1999) Oxygenation may improve with time during one-lung ventilation. Anesth Analg 89: 258-259.
37. Whynot S, Xu Z, Zhang H, Slutsky AS, Chappe V, et al. (2014) Protective ventilation attenuates lung injury by combined mechanisms of reduced mechanical stress and hypercapnia in a rat model of ARDS. Am J Respir Crit Care Med 189:A3100.
38. Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, et al. (2000) Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung perfusion. Am J Respir Crit Care Med 162: 2287-2294.
39. Bidani A, Tzouanakis AE, Cardenas VJ Jr, Zwischenberger JB (1994) Permissive hypercapnia in acute respiratory failure. JAMA 272: 957-962.
40. Laffey JG, O’Croinin D McLoughlin P, Kavanagh BP (2004) Permissive hypercapnia-Role in protective lung ventilatory strategies. Intensive Care Med 30:347-356.
41. Park SH, Jeon YT, Hwang JW, Do SH, Kim JH, et al. (2011) A preemptive alveolar recruitment strategy before one-lung ventilation improves arterial oxygenation in patients undergoing thoracic surgery: a prospective randomised study. Eur J Anaesthesiol 114:1025-1035.
42. Michelet P, D’Journo XB, Roch A, Doddoli C, Marin V, et al. (2006) Protective ventilation influences systemic inflammation after esophagectomy. Anesthesiology 105:911-919.
43. Licker M, Diaper J, Villiger Y, Spiliopoulos A, Licker V, et al. (2009) Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care 13:R41.
44. Yang M, Ahn HJ, Kim K, Kim JA, Yi CA, et al. (2011) Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 139:530-537.
45. Carraminana A, Ferrando C, Unzueta MC, Navarro R, Suarez-Sipmann F, et al. Rationale and study design for an individualized perioperative open lung ventilation strategy in patients on one-lung ventilation (iPROVE-OLV). J Cardiothorac Vasc Anesth 33:2492-2502.
46. Slinger P (2002) Fluid management during pulmonary resection. Ann Card Anaesth 5:220-224.
47. Turnage WS, Lunn JL (1993) Postpneumonectomy pulmonary edema: a retrospective analysis of associated variables. Chest 103:1646-1650.
48. Parquin F, Marchal M, Mehiri S, Herve P, Lescot B (1996) Post-pneumonectomy pulmonary edema: Analysis and risk facors. Eur J Cardiothorac Surg 10:929-932.
49. Evans RG, Naidu B (2012) Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury?. Interact Cardiovasc Thorac Surg 15:498-504.
50. Ahn HJ, Kim JA, Lee AR (2016) The risk of acute kidney injury from fluid restriction and hydroxyethyl starch in thoracic surgery. Anesth Analg 122:186-193.
51. Blank RS, Hucklenbruch C, Gurka KK, Scalzo DC, Wang XQ, et al. (2011) Intraoperative factors and the risk of respiratory complications after pneumonectomy. Ann Thorac Surg 92:1188-1194.
52. Thomas P, Michelet P, Barlesi F, Thirion X, Doddoli C, et al. (2007) Impact of blood transfusions on outcome after pneumonectomy for thoracic malignancies. Eur Respir J 29: 565-570.
53. Cerfolio RJ, Bryant AS, Thurber JS, Bass CS, Lell WA, et al. (2003) Intraoperative solumedrol helps prevent postpneumonectomy pulmonary edema. Ann Thorac Surg 76: 1029-1033.
54. Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, et al. (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354: 1671-1684.
55. Schilling T, Kozian A, Senturk M, Huth C, Reinhold A, et al. (2011) Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology 115:65-74.
56. Conno ED, Steurer MP, Wittlinger M, Zalunardo MP, Weder W, et al. (2009) Anesthetic-induced improvement of the inflammatory response to one-lung ventilation 110: 1316- 1326.
57. Schilling T, Kozian A, Kretzschmar M, Huth C, Welte T, et al. (2007) Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br J Anaesth 99: 368-375.
58. Frohlich D, Rothe G, Schwall B, Schmid P, Schmitz G, et al. (1997) Effects of volatile anaesthetics on human neutrophil oxidative response to the bacterial peptide FMLP. Br J Anaesth 78: 718-723. 59. Tschaikowsky K, Ritter J, Schroppel K, Kuhn M (2000) Volatile anesthetics differentially affect immunostimulated expression of inducible nitric oxide synthase. Anesthesiology 92: 1093-1102.
60. Nakazato K, Yoshida Y, Takemori K, Kobayashi K, Sakamoto A (2009) Expression of genes encoding drug-metabolizing enzymes are altered after sevoflurane, isoflurane, propofol or dexmedetomidine anesthesia. Biomed Res 30: 17-24.
61. Lumb AB, Slinger P (2015) Hypoxic pulmonary vasoconstriction. Anesthesiology 122: 932-946.
62. Basedovsky HO, Del Rey A (1996) Immune-neuro-endocrine interactions: Facts and hypothesis. Endocr Rev 17: 64-102. 63. Fares KM, Mohamed SA, Hamza HM, Sayed DM, Hetta DF (2014) Effect of thoracic epidural analgesia on proinflammatory cytokines in patients subjected to protective lung ventilation during Ivos Lewis esophagectomy. Pain Physician 17: 305-315.
64. Hou BJ, Du Y, Gu SX, Fan J, Wang R, et al. (2019) General anesthesia combined with epidural anesthesia maintaining appropriate anesthesia depth may protect excessive production of inflammatory cytokines and stress hormones in colon cancer patients during and after surgery. Medicine 98:e16610.
65. Su Y, Pu Y, Zhao Z, Yang X (2020) Influence of combined epidural anesthesia on cognitive function, inflammation and stress response in elderly liver cancer patients undergoing surgery. Oncol Lett 19: 2733-2738.
66. Okuda J, Suzuki T, Wakaizumi K, Kato J, Yamada T, et al. (2022 in press) Effects of thoracic epidural anesthesia on systemic and local inflammatory responses in patients undergoing lung cancer surgery: a randomized controlled trial. J Cardiothorac Vasc Anesth.
67. Li XQ, Tan WF, Wang J, Fang B, Ma H (2015) The effects of thoracic epidural analgesia on oxygenation and pulmonary shunt fraction during one-lung ventilation: a meta-analysis. BMC Anesthesiol 15: 166.
68. Wang JW, Wu AS, Wu Y (2017) Endothelial glycocalyx layer: a possible therapeutic target for acute lung injury during lung resection. Biomed Res Int 2017: 5969657.



