Review Article - (2025) Volume 4, Issue 1
A Review on New Advancement in AIFA Guidelines
2Department of Pharmaceutical Chemistry, SSS’s Divine College of Pharmacy, Nampur Road, Satana, Nashi, India
Received Date: Dec 02, 2024 / Accepted Date: Dec 26, 2024 / Published Date: Jan 04, 2025
Copyright: ©©2025 Kaveri S. Gholap, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Gholap, K. S., Pagar, P. S., Bachhav, R. L., Deore, R. K., Surana, K. R., et al. (2025). A Review on New Advancement in AIFA Guidelines. Biomed Sci Clin Res, 4(1), 01-09.
Abstract
Italy's pharmaceutical industry is governed and managed by the Italian Medicines Agency (AIFA). It publishes guidelines that offer thorough advice and rules for the creation, authorization, and post-marketing surveillance of pharmaceutical goods. These recommendations seek to promote patients' and healthcare providers' appropriate use of medications and medical equipment while also guaranteeing their efficacy, safety, and quality. Clinical trials, marketing authorization, pharmacovigilance, pricing, and reimbursement are only a few of the areas of pharmaceutical regulation that are covered by the AIFA standards. The recommendations seek to assure the availability of safe and effective medical products for the Italian people, foster innovation in the pharmaceutical business, and promote public health and safety by establishing defined standards and criteria.
Keywords
Agenzia Italiana Del Farmaco, AIFA, Guidelines, Italian Medicine Agency, COVID-19
Introduction
The Italian Medicines Agency (AIFA) has recently made significant modifications to the pharmaceutical criteria it employs for the evaluation and approval of medications. The objective of these advancements is to accelerate the assessment procedure while guaranteeing the effectiveness and safety of pharmaceutical products in Italy [1]. One of the main modifications to the guidelines involves a stricter and more comprehensive examination of clinical trial data. This involves placing particular emphasis on certain groups of patients and employing real-world data to substantiate the authorization of drugs. Implementing this measure would guarantee that a greater number of patients will receive the advantages of secure and effective treatment using medications that have been authorized for use in Italy [2]. The amended recommendations emphasize the importance of pharmacovigilance and post-marketing surveillance, as highlighted by the AIFA. This entails prioritizing post-market surveillance of pharmaceutical goods' safety and implementing risk management strategies for individual pharmaceuticals [3]. The new guidelines aim to enhance patients' access to more affordable prescriptions in Italy by streamlining the evaluation process for generic drugs. An expedited approval road has been established in Italy to facilitate the availability of generic pharmaceuticals in the market. This channel is reserved for drugs that meet certain standards [4].
The Italian medical institution AIFA is a public organization that operates under the leadership of the Ministry of Health and the oversight of the Ministries of Economy and Health. It adheres to the principles of transparency, efficiency, and autonomy [5]. AIFA engages in partnerships with the Regions, the Italian National Institute of Health (ISS), research institutes, hospitals, medical schools, patient associations, medical professionals, learned societies, and the manufacturing and distribution businesses [6]. The AIFA (Italian Medicines Agency) provides guidelines for drug development and registration, pharmacovigilance, cost, and reimbursement. These recommendations are based on established standards and methodologies to ensure the superior quality, safety, and effectiveness of pharmaceuticals [7].
In summary, the recent advancements in Italy's pharmaceutical standards will promote innovation and competition in the pharmaceutical industry, while also ensuring that patients can obtain safe and efficient therapies. These modifications align with AIFA's objective to enhance patient safety and public health through rigorous monitoring and evaluation of pharmaceutical products [8]. With the support and guidance of the Italian Ministry of Health, AIFA plays a crucial role in ensuring the effectiveness, safety, and quality of drugs available in the country [9].
The Italian Medicine Agency
The Italian Medicines Agency (AIFA) is the regulatory authority responsible for overseeing pharmaceuticals in Italy. This public entity operates autonomously, transparently, and in compliance with efficiency criteria. The Ministry of Health oversees it and the Ministries of Economy and Health monitor it. It engages in partnerships with patients' associations, healthcare professionals, the pharmaceutical industry, scientific associations, regional authorities, the National Institute of Health, research institutes, and distributors [10].
Institution and Statutes
• Article 48, Legislative Decree no. 269/2003 (Law 326/2003);
• Ministerial Decree 20 September 2004, no. 245 (as amended by Ministerial Decree 29 March 2012, no. 53);
• Regulation 31 October 2009.
The Mission
• Promote good health through medicines
• Set fair pharmaceutical policies and assure their consistent application nationwide
• Manage the value and cost of medicines
• Promote pharmaceutical research and development
• Demonstrate independence and leadership both at home and internationally
1. The Committees: The scientific authority and autonomy of the AIFA is supported by the activities of 2 Technical Scientific Committees consisting of experts of well-established experience.
2. Technical Scientific Committee: The CTS assumes the responsibilities previously assigned to the National Drug Evaluation Board at the Ministry of Health. It evaluates the national and European marketing authorization applications, provides a consultation opinion on them, and assigns a classification for reimbursement [11].
3. Prices and Reimbursement Committee: It negotiates pricing for pharmaceutical items that are paid by the National Health Service with pharmaceutical companies in accordance with open methods, deadlines, and procedures determined by the Interministerial Committee for Economic Planning [12].
Key Responsibilities of AIFA Include [13]
1. Regulatory Approval: AIFA evaluates and approves medicinal products before they can be marketed in Italy. This involves assessing the safety, efficacy, and quality of drugs through a rigorous review process.
2. Pharmacovigilance: AIFA monitors the safety of medicinal products once they are on the market. It collects and analyzes information on adverse drug reactions to ensure the ongoing safety of pharmaceuticals.
3. Price Regulation: AIFA plays a role in pricing and reimbursement decisions for medicinal products. It works to balance the need for access to effective treatments with cost considerations.
4. Clinical Trials Oversight: AIFA oversees and regulates clinical trials conducted in Italy, ensuring that they adhere to ethical and scientific standards.
5. Guidelines and Policies: The agency develops guidelines and policies to guide healthcare professionals, the pharmaceutical industry, and the public in matters related to medicinal products.Public Health Initiatives: AIFA may also be involved in public health initiatives related to medicines, providing information and education to healthcare professionals and the public. The Italian Medicines Agency (AIFA), the regulatory authority responsible for the regulation and oversight of medicinal products in Italy.
Key Aspects of AIFA's Medicinal Guidelines Include [14]
1. Marketing Authorization: AIFA is responsible for evaluating and granting marketing authorization for medicinal products before they can be marketed and sold in Italy.
2. Clinical Trials: AIFA oversees and regulates clinical trials conducted in Italy, ensuring they adhere to ethical standards and comply with applicable regulations.
3. Pharmacovigilance: AIFA monitors the safety of medicinal products on the market and collects information on adverse drug reactions, taking appropriate regulatory actions when necessary.
4. Pricing and Reimbursement: AIFAis involved in establishing pricing and reimbursement policies for pharmaceuticals in Italy, working to balance access to medicines with cost containment.
5. Guidelines and Recommendations: AIFA issues guidelines and recommendations related to various aspects of pharmaceuticals, including good manufacturing practices, pharmacovigilance, and specific therapeutic areas.
The AIFA (Italian Medicines Agency) Guidelines are Based on the Following Principles [15]
1. Transparency: The guidelines aim to be transparent in their decision-making process and provide clear, accessible information to healthcare professionals, patients, and the public.
2. Sound Scientific Evidence: The guidelines are based on the best available scientific evidence and aim to promote the use of safe and effective medicines.
3. Patient-Centered Care: The guidelines prioritize the needs and preferences of patients and aim to promote patient- centered care and shared decision-making between healthcare professionals and patients.
4. Ethical Considerations: The guidelines adhere to ethical principles and considerations, including respect for patient autonomy, beneficence, and non-maleficence.
5. Accessibility: The guidelines aim to be accessible to all stakeholders, including healthcare professionals, patients, and the public, and provide clear and understandable information.
6. Continuous Review and Update: The guidelines are regularly reviewed and updated to reflect the latest scientific evidence and best practices in medicine.
7. Collaboration and Engagement: AIFA collaborates with healthcare professionals, patients, and other stakeholders to develop and implement theguidelines, ensuring that they are relevant and effective in clinical practice.
The Specific Protocols Outlined in AIFA Guidelines Include [16]
1. Clinical Trial Protocols: Provide guidance on the design, conduct, and reporting of clinical trials to ensure that they are conducted ethically and generate reliable data on the safety and efficacy of medicines.
2. Pharmacovigilance Protocols: Detail the procedures and responsibilities for the monitoring and reporting of adverse drug reactions to ensure the ongoing safety of medicines on the market.
3. Pricing and Reimbursement Protocols: Outline the criteria and procedures for the evaluation of new medicines to determine their pricing and reimbursement status.
4. Marketing Authorization Protocols: Define the requirements and procedures for obtaining marketing authorization for new medicines, including the submission of comprehensive documentation on their safety, efficacy, and quality.
5. Quality Control Protocols: Provide standards and protocols for the quality control of medicines, including manufacturing processes, storage, and distribution, to ensure that they meet regulatory requirements.
These protocols are designed to ensure that medicines meet regulatory standards for safety, efficacy, and quality, and that they are appropriately monitored and managed throughout their lifecycle [17]. Working with AIFA guidelines in the pharmaceutical industry in Italy involves ensuring compliance with the regulations and requirements set forth by the Italian Medicines Agency (AIFA). Here are the key steps for working with AIFA guidelines in the pharmaceutical industry:
1. Familiarize Yourself with AIFA Guidelines: It is essential for pharmaceutical companies to thoroughly understand the AIFA guidelines, whichencompass various aspects such as clinical trials, marketing authorization, pharmacovigilance, pricing and reimbursement, and quality control. These guidelines are available on the AIFA website or through official publications.
2. Obtain Regulatory Approvals: If a pharmaceutical company intends to conduct clinical trials in Italy, obtain marketing authorization for a new medicine, or market existing pharmaceutical products, it must navigate and adhere to AIFA guidelines to obtain the necessary regulatory approvals. This involves submitting comprehensive documentation and complying with the specific requirements outlined by AIFA.
3. Comply with Pharmacovigilance Requirements: Pharmaceutical companies must follow AIFA's pharmacovigilance protocols for monitoring adverse drug reactions, collecting and reporting safety data, and implementing risk management plans and post-marketing surveillance as per AIFA guidelines.
4. Pricing and Reimbursement: AIFA guidelines outline the evaluation and pricing criteria for new medicines to be reimbursed in Italy. Pharmaceutical companies need to understand and ensure compliance with these guidelines when seeking reimbursement for their products.
5. Quality Control and Manufacturing Standards: AIFA sets standards and protocols for the quality control of pharmaceutical products, including manufacturing processes, storage, and distribution. Companies need to ensure that their manufacturing practices and product quality align with AIFA guidelines
6. Maintain Ongoing Compliance: Once pharmaceutical products are on the market, companies must continue to adhere to AIFA guidelines, including reporting and compliance with updated regulations and requirements [18,19].
AIFA (Italian Medicines Agency) guidelines have a significant impact on the pharmaceutical industry in Italy. The guidelines are designed to regulate and ensure the safety, efficacy, and quality of pharmaceutical products. Below are some of the effects of AIFA guidelines on the pharmaceutical industry [20].
1. Regulatory Compliance: Pharmaceutical companies must adhere to AIFA guidelines to obtain regulatory approvals for clinical trials, marketingauthorization, and post-marketing activities. Compliance with these guidelines is mandatory and ensures that pharmaceutical products meet the required standards.
2. Pharmacovigilance: AIFA guidelines mandate strict pharmacovigilance requirements for monitoring and reporting adverse drug reactions. This ensures that pharmaceutical companies maintain high standards of drug safety and promptly report any adverse events associated with their products.
3. Pricing and Reimbursement: AIFA guidelines govern the evaluation and pricing of pharmaceutical products to be reimbursed by the Italian National Health Service. Pharmaceutical companies must comply with these guidelines to secure reimbursement for their products.
4. Quality Control and Manufacturing Standards: AIFA guidelines dictate the quality control requirements for pharmaceutical manufacturing processes, ensuring that products meet specific standards for safety, efficacy, and quality. Compliancewith these standards are essential for ensuring the reliability and safety of pharmaceutical products.
5. Market Access: AIFA guidelines play a crucial role in market access for pharmaceutical products. Compliance with the guidelines is necessary for obtaining marketing authorizations and ensuring that products are available on the Italian market.
6. Patient Safety: Ultimately, the AIFA guidelines aim to safeguard public health by ensuring that pharmaceutical products meet stringent safety and efficacy standards. Compliance with these guidelines helps to protect patients and maintain trust in the pharmaceutical industry.
AIFA Simplified the Procedure for Applications of New MA for Generic and Hybrid Medicines
The Italian Medicines Agency (AIFA) announced on May 30, 2023, that a new simplified procedure will be implemented to expedite the relevant registration process for applications for new MAs under national, mutual recognition, and decentralised procedures submitted under Articles 10(1) (generic medicines) and 10(6) (hybrid medicines) of Legislative Decree No. 219/2006. This change will take effect starting with the Scientific Technical Committee (STC) meeting in June 2023 [21].
The first Italian guidelines for the economic evaluation of health technologies aim to provide applicants with detailed information on how to complete the economic chapter of the P&R dossier. They were initially presented with reference to the Inter-ministerial Committee for Economic Planning (CIPE) decision dated 1 February 2001 [22].
Afterwards, on the 2nd of August 2020, a decree presenting general indication on new requirements of P&R dossier abrogated the 2001 legislative framework. The new detailed guidelines for the completion of P&R dossier were first made available for public consultation on the 16th of September 2020, being published in their final version on the 30th of December 2020. In this version, guidelines indicate that, for new technologies, an economic analysis is expected or its absence should be properly justified. Nonetheless, if this is missing, AIFA retains the right of requiring it, leading to the suspension of the negotiation process. The economic analysis should be presented in section E and Appendix 2.2 of the P&R dossier and should be filled following May’s guidelines [23]. A summary of the recent relevant changes in the Italian HTA process is presented in Table 1, while other comparison elements are resumed in Table 2.
AIFA: Agenzia Italianadel Farmaco; CIPE: Committee for Economic Planning; P & R: price and reimbursement
|
Date |
Event |
Notes |
|
May 2020 |
AIFA publishes on its website the new guidelines for or the economic evaluation of health technologies in Italian. |
Guidelines are framed within the Inter-ministerial CIPE decision dated 1 February 2001 |
|
July 2020 |
AIFA publishes on its website the new guidelines for the economic evaluation of health technologies in English |
Guidelines are framed within the Inter-ministerial CIPE decision dated 1 February 2001 |
|
2nd August 2020 |
Decree law abrogating CIPE 2001 |
The decree lists the information that should be included in the request of P & R dossier |
|
16th of September |
AIFA publishes on its website provisional guidelines and template for the completion of P&R dossier for public consultation |
In the new provisional template, the economic evaluation in terms of cost-effectiveness analysis is not identified as a compulsory element to be submitted |
|
30th of December |
AIFA publishes on its website final guidelines and template for the completion of P & R dossier |
Guidelines are framed within the decree law of 2nd of August 2020 |
Table 1: Resume of the Legislative Framework of Italian Guidelines for Economic Assessment
AIFA: Agenzia Italiana del Farmaco; EQ-5D: EuroQol-5D (EQ-5D); HRQoL: HealthRelated Quality of Life; MAH: Marketing Authorization Holders; NHS: National Health System; NICE: National Institute of Health and Care Excellence; QALYs: Quality Adjusted Life Years; TA: Technology appraisal; PSS: Personal Social Services; RCTs: Randomized Clinical Trials.
|
c |
Italy |
England |
|
Application |
MAH should present a cost-effectiveness analysis for- a) orphan medicines; b) new active substances c) new therapeutic indications of established products |
TA are produced for interventions that are likely to have: a significant health benefit; b) significant impact on other health-related government policies: c) significant impact on NHS resources: d) significant variations in the use of the technology; e) national guidance is likely to add value |
|
Time horizon |
AIFA suggests considering three-time horizons (5 years, 10 years and lifetime) No preferred one is stated, it should anyway be a period long enough to capture all the differences between the compared alternatives |
It should be long enough to capture the whole impact that interventions have on the health and associated costs of the population assessed |
|
Discounting |
Both costs and health effects al an annual rate of 3.0% Sensitivity analysis discount rates of 0.0% and 5.0% |
Both costs and health effects al an annual rate of 3.5% Sensitivity analysis discount rates of 1.5% |
|
Population |
As specified in marketing authorization |
As specified in marketing authorization |
|
Comparators |
MAH should justify the selection of the comparator based on the national or international guidelines the national clinical practice or the lack of valid alternative |
NICE identifies all potentially relevant comparators during the scoping process. at the beginning of the appraisal process, by taking into account the natural history of the disease, NICE guidance. cost-effectiveness analyses, and available comparators |
|
Perspective of the analysis |
NHS perspective is recommended for the base case analysis Societal perspective may be presented |
Perspective of the NHS and Pss |
|
Health effects |
RCTs as preferred evidence Other sources of evidence not excluded but should be accurately described in terms of study methods assumptions and results No methodological requirements for evidence synthesis |
RCTs as preferred evidence of other sources of information are used, biases should be quantified and adjusted for Data should be presented in network meta analyses if evidence available in non head-to-head RCTs |
|
Benefit and Measurement |
Use referring to the Italian context if possible. If multiple altemative data sources are available, the resulting uncertainty should be assessed in : sensitivity analysis |
Preferably expressed in QALYs and measured with the EQ-SD. When possible, HRQoL associated to health states should be measured in patients while general population should be engaged to elicit preferences |
|
Sensitivity analysis |
Probabilistic sensitivity analyses result should be graphically represented in scatter plus and acceptability curves |
Probabilistic sensitivity analyses are preferred graphically represented in scatter plots and acceptability curves and tabulated report on correlation/independence between parameters should be examined |
Table 2: Resume of Differences and Similarities Between Italian and English Guidelines
Drug Prescriptions in the Outpatient Management of COVID-19 Evidence- Based Recommendations Versus Real Practice
Italy quickly became one of the most affected regions in the globe after becoming the first nation in Europe to diagnose coronavirus disease-19 (COVID-19) in humans. As of March 15, 2020, the nation had recorded over 22,500 cases and 1.625 deaths from COVID-19, marking the start of one of the biggest and most dangerous clusters in the world. Rumours spread by the media, articles published without peer review, and limited clinical trials encouraged doctors to prescribe a large number of off-label treatments during the peak of the epidemic. Put another way, doctors searched for medications that were ready to use regardless of thequality of the data that was available and that was backed by strong assumptions about their prospective benefits. Treatment for COVID-19 has been recommended and even prescribed for patients since the outbreak began. This includes corticosteroids, high-dose vitamin C, and vitamin D, antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), antithrombotic, antivirals, and many other unnecessary medications like biologics. Nevertheless, these uses are not authorised, and the drugs are being used off-label. For pharmacological treatments with either suspected or confirmed COVID-19 patients in the outpatient context, the majority of the recommendations released during the first pandemic stage lack evidence from randomised clinical trials. The majority of COVID- 19-positive patients did not require hospitalisation since they were either asymptomatic or had mild to moderate symptoms. Nonetheless, clinical data regarding outpatient care in the early stage of the infection is still scare, and several factors can influence the clinician choice such as sociodemographic and professional factors and perceived disease severity. Additionally, during the various stages of the pandemic, government messages and media coverage regarding specific pharmaceutical therapy swayed public opinion, leading some to believe thatthesedrugs were risky or irreversible when used to treat COVID-19 [24,25].
In order to fill this vacuum, the Italian Medicines Agency (AIFA) and a scientific committee collaborated to produce a two-page card on particular outpatient drug treatments. The card included information on the safety concerns or use restrictions, as well as a brief methodological analysis and the rationale and data supporting the drug's use (Addis t al., 2020). A caution against routine use of certain medications and their combinations outside of clinical trials was also issued by the agency, so creating a gap between clinical research and clinical practice (Figure 1). According to the Italian Medicines Agency (AIFA), 2021, the information cards were updated on a regular basis to reflect new findings and were accessible to the public on the AIFA website [26,27].
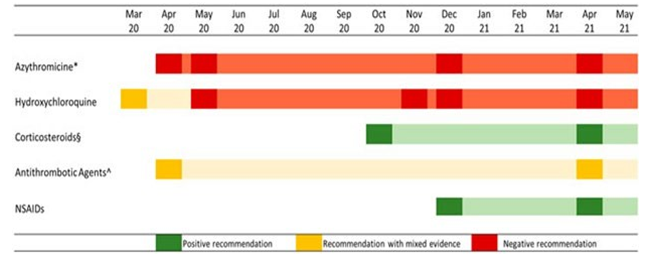
Figure 1: AIFA Recommendations on the Use of Specific Drug Categories in the Outpatient Treatment of COVID 19 Cases
Moreover, vitamin D use in COVID-19 patients was suggested by the Italian Society of General Medicine and Primary Care (SIMG), although AIFA did not consider it for outpatient treatment because of limited evidence. The aim of this study was to measure the use of outpatient drug treatments in COVID-19-positive patients in a real practice setting, taking into account the Evidence-Based Medicine (EBM) recommendations issued by a central competent authority. Independent of AIFA recommendations, the use of drug therapy in the management of outpatient COVID-19 cases was frequent (about one-third of the cases). The most used drug therapy was antibiotics, specifically azithromycin, despite thenegative recommendation of AIFA, while the use of corticosteroids increased after the positive recommendation of regulatory agency for the use in subjects with severe COVID-19 disease. The use of hydroxychloroquine was limited to the early pandemic period where evidence on its potential benefit was controversial. Antithrombotics were widely used in outpatient settings, even if their use was recommended for hospitalized patients [28,29].
Clinical Research Activities During COVID-19: The Point of View of a Promoter of Academic Clinical Trials
During the COVID-19 emergency, IRST IRCCS, an Italian cancer research institute and promoter of no profit clinical studies, adapted its activities and procedures as per European and national guidelines to maintain a high standard of clinical trials, upholdparticipant safety and guarantee the robustness and reliability of the data collected. This study presents the measures adopted by our institute with the aim of providing information that could be useful to other academic centers promoting clinical trialsduring the pandemic. Italian legislation no. 23, art. 40, of April 8, 2020 established urgent regulations for pharmacological clinical trials during the Coronavirus Disease 19 (COVID-19) emergency. The Italian Medicines Agency the Italian competent authority for pharmacological clinical trial evaluation and, in particular, for studies on patients with COVID-19 , published several press releases on the matter. The most recent was that of April 7, 2020, which provided indications for the management of clinical trials and of substantial trial amendments following the exceptional restrictive measures introduced by the Italian government to cope with the pandemic. The measures adopted were to be valid until further notice and were closely linked to the state of emergency approved by the Council of Ministers on January 31, 2020 [30,31].
On April 28, 2020, the European Medicines Agency (EMA) published specific guidelines establishing simplification measures to be implemented during the public health crisis until their revocation by the European Union/European Economic Agreement (EU/EEA). European and Italian guidelines have promoted pragmatic and harmonized actions capable of guaranteeing the flexibility and procedural simplifications needed to maintain the integrity of clinical trials, the rights, safety and wellbeing of trial participants, and the safety of clinical trial staff. In 2012, IRST was formally recognized by the Italian Ministry of Health as a Scientific Institute for Research, Hospitalization and Healthcare (IRCCS). The institute promotes, coordinates and manages academic trials that arise from the innovative ideas of its researchers [32].
In Italy, non-profit trials are managed according to the ministerial decree of December 17, 2004. This legislation stipulates the general requirements and conditions for the execution of pharmacological clinical trials, in particular those on the improvement of clinical practice, as an integral part of healthcare. The legislation focuses on clinical trials whose promoter is a no profit organization and as such cannot be a marketing authorization holder. The ownership of the data pertaining to the trial, its execution and the results obtained belong to the promoter and the clinical trial cannot be profit-based or used for the industrial development of a drug. If these conditions are met, drug costs are paid by the National Health Service, Ethical Committee and Competent Authority fees are waived, and there are economic (i.e. minimum insurance requirements) and organizational benefits [33].
During the COVID-19 pandemic, clinical trial participants were in self-isolation/quarantine, with limited access to public institutes because of the risk of the spread of infection. Medical staff involved in clinical studies was also engaged in critical tasks related to the containment of the virus. Within this context, IRST, as a promoter of no profit studies, adapted its activities and procedures in accordance with European and national guidelines in an effort to maintain a high standard of clinical trials. Exceptional measures were implemented and trials were amended [34].
Some measures adopted by IRST could also be effective outside of the COVID-19 period, e.g. numerous activities relating to clinical trial management could be performed on a home-working basis, using suitable digital technologies. In the future, electronic medical records and shared guidelines will be essential for the correct identification and management of trial risks, including the protection of the rights and privacy of subjects taking part. Promoter supervision could be increased by implementing centralized monitoring tools to guarantee data quality. Closer collaboration between promoters and local study staff is needed to optimize trial management [35].
Eligibility Criteria for Pediatric Patients who May Benefit from Anti SARS-CoV-2 Monoclonal Antibody Therapy Administration: An Italianinter-Society Consensus Statement The fast diffusion of the SARS-CoV-2 pandemic together with the increasing knowledge of the virus have called for an equally rapid evolution of the therapeutic options evaluated to deal with this new pathogen. During the last two years, the scientific community has made an unprecedented effort investing enormous resources in the development of new therapies and vaccines against SARS-CoV-2. Human recombinant monoclonal antibodies (mAbs) have recently been approved by the Food and Drug Administration (FDA) for the emergency use in subjects infected with SARSCoV-2 and with specific risk factors [36].
Both those drugs’ combinations act in the same way targeting epitopes of the Receptor Binding Domain (RBD) of the SARS- CoV-2’s spike protein, which represents the main antigen of the virus. This prevents the connection between the pathogen and the Angiotensin Converting Enzyme type 2 (ACE-2) receptors, blocking the spread into the host’s cells and causing the opsonisation of the virus [37].
The rapid development of these new molecules has been accompanied by poor data on their utilization in adults and by a lack of studies regarding efficacy and safety in paediatric patients. Notably, some studies in vitro have showed a limitation in control of replication of the viral variants and a growing risk of developing new variants. However, despite the limited data regarding these drugs, those seem to guarantee a favourable security and efficacy profiles in adult patients in the initial phase of infection (with mild or moderate symptoms and high viral loads) for which we still need a reliable therapeutic option. To date no studies about the efficacy and the safety of anti SARS-CoV-2 monoclonal antibodies in paediatric patients have been published. In addition, infected paediatric patients have often showed a favourable outcome regardless of the presence of risk factors or comorbidities. For these reasons, their utilization is still under debate calling for a judicious and detailed use [38].
Regarding these considerations and the risk of an increased diffusion of COVID-19, as Italian Society of Paediatrics and other paediatric subspecialist scientific society, we try to detail the indications for the use of mAbs in the paediatric population, upon the available evidence from the scientific literature and experts’ recommendation. Therefore, FDA and AIFA have allowed those drugs to be used also in the paediatric patients limited to subjects aged at least 12 years, with recently diagnosed SARS-CoV-2 infection and mild or moderate symptoms, in presence of specific risk factors for an higher risk of progression to severe disease [39].
Those risk categories are derived from the existing literature which reports an increased risk for severe COVID-19 in children with comorbidities. However the studies analyzing the determinants for progression to severe disease are mainly monocentric, with limited numbers and reporting mostly generic risk categories
In particular, in a meta-analysis on 285,000 paediatric patients infected by SARS-CoV-2, children with comorbidities had a relative risk ratio of severe infection and mortality respectively of 1.79 and 2.81 more than those without comorbidities. Nevertheless, the relative low numbers of the children with the different comorbidities precluded an analysis of the relative risk (RR) for single underlying condition, except for obesity.
The Panel of experts from the Scientific Societies involved in this Consensus remarks that, to date, there are no sufficiently solid data regarding neither the higher risk of progression to severe COVID-19 infection nor the efficacy in preventing that progression with the use of anti SARS-CoV2 monoclonal antibodies in children with chronic diseases. Moreover, in the wide majority of cases, the most severe presentations in children and adolescents are represented by the so-called Multisystem Inflammatory Syndrome in Children (MIS-C) mostly occurring 3–6 weeks after SARS-CoV-2 infection in patients without significant comorbidities except, in some cases, obesity.
Lessons Learned from COVID-19 for Clinical Research Operations in Italy: What Have we Learned and what Can We Apply in the Future?
The coronavirus disease 2019 (COVID-19) pandemic has stressed the importance of health research as never before. In the specific domain of clinical research, the effort to rapidly find responses to health challenges and therapeutic hypotheses has highlighted the need for efficient, timely, ethically correct research. The guidelines published by the Agenzia Italianadel Farmaco have shown that some useful changes are feasible: simple and rapid methods have been implemented to conduct clinical research in the emergency conditions of the pandemic, maintaining high levels of quality. In this perspective, four Italian scientific associations operating in clinical research have worked together to evaluate which measures, among the ones implemented during the pandemic, have been particularly significant and potentially effective under normal conditions or in case of emergencies, and that therefore will be useful in the future as well.
Conclusion
In conclusion, the new advancements of AIFA guidelines reflect the continuous effort to improve the quality and safety of medical products in Italy. The updated guidelines emphasize the importance of transparency, data integrity, and risk-based approach in the evaluation and authorization process for pharmaceuticals, medical devices, and other related products. The incorporation of new strategies and technologies in the guidelines will likely enhance the efficiency of the approval process, resulting in faster delivery of safe and effective products to the market. Overall, the new advancements demonstrate AIFA's commitment to keeping pace with the rapidly evolving landscape of healthcare and regulatory standards, ultimately benefiting the public and healthcare industry in Italy. Overall, the advancements in AIFA’s guidelines have significantly improved the regulatory framework for medicinal products in Italy. These updates have enhanced the efficiency and transparency of the approval process, strengthened the monitoring of drug safety, and promoted the appropriate use of pharmaceutical products. As a result, patients in Italy can have confidence in the safety and effectiveness of the medicines they use, while pharmaceutical companies can benefit from a more predictable and streamlined regulatory environment.
References
1. Villa, F., Jommi, C., Genazzani, A., Antignani, S., Montilla, S., & Melazzini, M. (2018). Early access to the market: from conditional EMA approvals to AIFA's special negotiation agreements. GLOBAL & REGIONAL HEALTH TECHNOLOGY ASSESSMENT.
2. Wang, H., Jin, X. Y., Pang, B., Liu, C. X., Zheng, W. K., Yang, F. W., ... & Zhang, J. H. (2020). Analysis on clinical study protocols of traditional Chinese medicine for coronavirus disease 2019. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China journal of Chinese materia medica, 45(6), 1232-1241.
3. Cheng, M. P., Lee, T. C., Tan, D. H., & Murthy, S. (2020). Generating randomized trial evidence to optimize treatment in the COVID-19 pandemic. Cmaj, 192(15), E405-E407.
4. Addis, A., Genazzani, A., Trotta, M. P., Magrini, N., & Italian Medicines Agency's Scientific Committee and COVID-19 Crisis Unit. (2020). Promoting better clinical trials and drug information as public health interventions for the COVID-19 emergency in Italy. Annals of Internal Medicine, 173(8), 654- 655.
5. Aher, P., Surana, K., Ahire, E., Patil, D., Sonawane, D., & Mahajan, S. (2023). Development and validation of RP-HPLC method for quantitative determination of 4-amino benzene sulphonamide in sulphonamide hydrochloride. Trends in Sciences, 20(6), 5209-5209.
6. Carley, S., Horner, D., Body, R., & Mackway-Jones, K. (2020). Evidence-based medicine and COVID-19: what to believe and when to change. Emergency Medicine Journal, 37(9), 572-575.
7. Yeola, C. A., Sonawane, V. N., Sonawane, V. N., Surana, K. R., Patil, D. M., & Sonawane, D. D. (2023). Development and Validation of Simple UV-Spectrophotometric Method for Estimation of Diclofenac Sodium. Asian Journal of Pharmaceutical Analysis, 13(3), 183-189.
8. Vitali, L., Merlini, A., Galvagno, F., Proment, A., & Sangiolo, D. (2022). Biological and exploitable crossroads for the immune response in cancer and COVID-19. Biomedicines, 10(10), 2628.
9. Sonawane, V. N., Yeola, C. A., Sonawane, V. N., Surana, K. R., Patil, D. M., & Sonawane, D. D. (2023). Estimation of Paracetamol in various brands of Paracetamol Tablets and their Comparative Study. Asian Journal of Pharmaceutical Analysis, 13(3), 155-161.
10. Payne, J. D., Sims, K., Peacock, C., Welch, T., & Berggren, R. E. (2021, July). Evidence-based approach to early outpatient treatment of SARS-CoV-2 (COVID-19) infection. In Baylor University Medical Center Proceedings (Vol. 34, No. 4, pp. 464-468). Taylor & Francis.
11. Jacobsen, K. H. (2020). Will COVID-19 generate global preparedness?. The Lancet, 395(10229), 1013-1014.
12. Scarcia, M., Ludovico, G. M., Fortunato, A., & Fiorentino, A. (2020). Patients with cancer in the COVID-19 era: the clinical trial issue. Tumori Journal, 106(4), 271-272.
13. Bolislis, W. R., De Lucia, M. L., Dolz, F., Mo, R., Nagaoka, M., Rodriguez, H., ... & Kühler, T. C. (2021). Regulatory agilities in the time of COVID-19: overview, trends, and opportunities. Clinical therapeutics, 43(1), 124-139.
14. Ahire, E. D., Sonawane, V. N., Surana, K. R., Jadhav, K. R., Sonawane, D. D., & Shah, A. A. (2020). Convalescent plasma therapy: A promising approach in the treatment of Covid-19. Int J Pharm Sci Res, 11, 4078-4086.
15. Surana, K. R., Savale, L. V., Aher, J. S., Aher, S. N., Sonawane, D. D., & Patil, D. M. (2023). Nutraceuticals for the COVID-19 Prevention and Treatment. International Journal of Advanced Biological and Biomedical Research, 11, 247-260.
16. Valmorri, L., Vertogen, B., Zingaretti, C., Miserocchi, A., Volpi, R., Clemente, A., ... & Nanni, O. (2021). Clinical research activities during COVID-19: the point of view of a promoter of academic clinical trials. BMC medical research methodology, 21(1), 91.
17. Sim, A. J., Redler, G., Peacock, J., Naso, C., Wasserman, S., McNitt, K. B., ... & Rosenberg, S. A. (2020). Harnessing COVID-driven technical innovations for improved multi- disciplinary cancer care in the post-COVID era: the virtual patient room. Cancer Control, 27(1), 1073274820964800.
18. Parab, A. A., Mehta, P., Vattikola, A., Denney, C. K., Cherry, M., Maniar, R. M., & Kjaer, J. (2020). Accelerating the adoption of eSource in clinical research: a transcelerate point of view. Therapeutic innovation & regulatory science, 54, 1141-1151.
19. Cagnazzo, C., Testoni, S., Guarrera, A. S., Stabile, S., Taverniti, C., Federici, I., ... & Monti, M. (2019). Clinical research coordinators: a crucial resource. Recenti Progressi in Medicina, 110(2), 65-67.
20. Harris, A. L. (2020). COVID-19 and cancer research. British Journal of Cancer, 123(5), 689-690.
21. Biopharma, T. (2020). Beyond COVID-19: Modernizing Clinical Trial Conduct.
22. Tuccori, M., Convertino, I., Ferraro, S., Cappello, E., Valdiserra, G., Focosi, D., & Blandizzi, C. (2020). The impact of the COVID-19 “Infodemic” on drug-utilization behaviors: implications for pharmacovigilance. Drug safety, 43, 699-709.
23. Shojaei, A., & Salari, P. (2020). COVID-19 and off label use of drugs: an ethical viewpoint. DARU Journal of Pharmaceutical Sciences, 28, 789-793.
24. Livingston, E., & Bucher, K. (2020). Coronavirus disease 2019 (COVID-19) in Italy. Jama, 323(14), 1335-1335.
25. Douglas, M., Moy, S., & Hernandez, N. (2021). Impact of COVID-19 on outpatient antimicrobial prescribing patterns in New York City. Infectious Diseases in Clinical Practice, 29(6), e352-e355.
26. Sanders, J. M., Monogue, M. L., Jodlowski, T. Z., & Cutrell, J. B. (2020). Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama, 323(18), 1824-1836.
27. Ammassari, A., Di Filippo, A., Trotta, M. P., Traversa, G., Pierantozzi, A., Trotta, F., & Magrini, N. (2021). Comparison of demand for drugs used for COVID-19 treatment and other drugs during the early phase of the COVID-19 pandemic in Italy. JAMA network open, 4(2), e2037060-e2037060.
28. Diazâ?ÂÂArocutipa, C., Brañezâ?ÂÂCondorena, A., & Hernandez, A. V. (2021). QTc prolongation in COVIDâ?ÂÂ19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: a systematic review and metaâ?ÂÂanalysis. Pharmacoepidemiology and Drug Safety, 30(6), 694-706.
29. Cafiero, D., Giugliano, S., Putignano, D., & Lopatriello, S. (2020). PNS94 The AIFA Assessment for Innovation Status: An Overview of 2017-2019 Reports. Value in Health, 23, S657-S658.
30. Marchesi, E., Monti, M., Campora, S., Gentili, G., Frati, P., Pirondi, S., ... & Cagnazzo, C. (2016). AIFA Determination 809/2015 on Phase I Clinical Trials: a new challenge for Italian Research. Annals of Oncology, 27, iv112.
31. Crippa, L., Bortone, L., Ferrari, E., Peirasso, G., Rondi, R., & Tria, V. (2022). POSC213 AIFA Assessments of Inclusion's Request in 648/96 List from January 2017 to June 2021. Value in Health, 25(1), S149.
32. Patil, S. J., Surana, K. R., & Mahajan, S. K. (2024). Quantification of active phytoconstituents in ethanolic extract of Mentha piperita by modern analytical tools. Res. Jr. Agril. Sci, 15(3), 615-621.
33. Ciresi, A., Cicciò, F., Amato, M. C., & Giordano, C. (2015). Revaluation of the clinical and metabolic behavior of children with isolated growth hormone deficiency during GH treatment according to newly proposed note 39 of the Italian Medicines Agency (AIFA). Journal of Endocrinological Investigation, 38, 1301-1307.
34. Fakir, J., Surana, K. R., Patil, D. M., & Sonawane, D. D. (2023). Survey Based Assessment of Adverse Effect in Covid-19 Vaccination Breakthrough Infections.
35. Di Tonno, D., Martena, L., Taurisano, M., Perlin, C., Loiacono, A. C., Lagravinese, S., ... & Argentiero, A. (2024). The Requirements of Managing Phase I Clinical Trials Risks: The British and Italian Case Studies. Epidemiologia, 5(1), 137-145.
36. Marchesi, E., Monti, M., Nanni, O., Bassi, L., Piccinni- Leopardi, M., & Cagnazzo, C. (2018). New requirements for phase I trials: A challenge for Italian clinical research. Tumori Journal, 104(1), 15-21.
37. Sonawane, V. N., Suryawanshi, K. G., Wagh, K. H., Sonawane, S. L., Sonar, A. D., Sakle, S. J., & Sonawane, D. D. (2023). A Comparative Study of Dissolution Profiles on Various Brands of Diclofenac Sodium Prolonged Release Tablet Formulation. Prog. Chem. Biochem. Res, 6(4), 341-355.
38. Bonini, S., del Zompo, M., Francavilla, L., Garaci, E., Garattini, S., Liberati, A., ... & Trotta, F. (2009). Feasibility and challenges of independent research on drugs: the Italian medicines agency (AIFA) experience. European Journal of Clinical Investigation, 40(1), 69-86.
39. Fiorentino, F., & Urbinati, D. (2021). First Italian guidelines for the economic evaluation of health technologies: how do they compare to NICE standards. GIHTAD, 14(5), 1-6.



