Review Article - (2022) Volume 7, Issue 8
The truth and dispute in TNM staging, standardized care and evidence-based medicine in cancer management
Received Date: Jul 18, 2022 / Accepted Date: Jul 23, 2022 / Published Date: Aug 10, 2022
Copyright: ©Copyright: ©2022: Kangla Tsung, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Kangla Tsung, Zhangxu, Zhanghui, TANLUN Clinical Research Participants (2022) The truth and dispute in TNM staging, standardized care and evidence-based medicine in cancer management. Journal of Clinical Review & Case Reports 7(8):103-112
Abstract
Current clinical management of cancer is based on a three-pillar system: TNM staging, standardized care and evidence-based medicine. Together, they support the entire system of cancer management. In the system, there are more than 30 million cancer patients, more than a million medical workers taking care of these patients, and millions more research scientists looking for ways to conquer cancer, the emperor of all maladies. Everyone in this system is supposed to follow a number of principles derived from these three pillars. The net result, after decades of building and polishing, is the presence of a global system with massive infrastructure, advanced technologies, tremendous funding, monopolized discoursing platform of public opinion and talented people, all together to strengthen the few principles derived from the three pillars. It is almost impossible to doubt or challenge the correctness and usefulness of these three pillars and thus the principles derived from them, less to say to replace them with something different. Yet going back the history of these pillars, one may ask some very reasonable questions about their correctness. For example, patients with the same TNM staging do not have similar clinical outcome when treated by the same therapy. If they are same, why they respond differently to the same therapy? And if they are truly different, why are they treated by the same therapy? What makes the difference among them? What do results from clinical trials comparing groups of different patients really mean? Evidence-based medicine emphasizing “evidence”, but there is no evidence to show that putting every one with similar TNM staging to the same treatment plan is the best option for them. Medicine, like other branches of science, should emphasize reasoning, but this has not been the case in cancer management in which one only needs to pay attention to data but not why it is the way it is. On the other hand, we have findings from animal tumor models translated to clinical study in a totally unmatched way in which findings from individualized animal model are translated to a mixed group of patients. This always yielded failures. There is nothing wrong to do individualized research in human cancer patients as long as we realize its limitation and applicability to other cases. With this approach, we have accumulated some very interesting observations, developed some reliable principles from these individualized studies and have put these principles into a novel guiding system that helps to derive individualized treatment plans for any patient at any stage in their disease course. Here we present logic arguments that the three pillars of the current clinical practice in cancer management have severe shortcomings that need to be replaced with a more reasonable and individualized management system.
Keywords
Cancer, Clinical Management, TNM staging, Standardized care, Evidence-based medicine, Anti-tumor immunity, Mode of tumor replication, Individualized medicine, Cancer therapy, ICI Therapy
Introduction
The Three Pillars of Current Cancer Management
Current cancer management is based on a comprehensive platform supported by three pillars. These three pillars are TNM-based disease staging, standardized clinical care and evidence-based clinical research (also called evidence-based medicine). TNMbased cancer staging assigns each cancer patient with a designated stage reflecting the seriousness of their cancer. Apparently, the current TNM staging is evolved from an old disease measurement of looking only the size and invasiveness of the primary tumor, which, though intuitive and often correct, only correctly predicted the seriousness of a cancer case in some time. With more clinical observations accumulated, it was realized that the presence of local and distant metastasis is a much more accurate indication of the prognosis of a disease than the size and invasiveness of the primary tumor. Thus eventually, a comprehensive score combining the characteristics of primary tumor and the number and location of metastasis took shape forming the current TNM staging system [1,2]. After several decades, this TNM staging system has become the most important basis for cancer management with several principles and practices derived from it. For example, entire cancer imaging analysis is built on the need for TNM designation. Any treatment selection is also based on the TNM designation for each cancer case. This brings in the second pillar of cancer management, standardized care according to an established guideline. The guideline is designed according to the TNM staging for that type of cancer. Selection of treatment plan looks into the TNM staging of each patient and the guideline prepared for that staging of that cancer. Then how is certain treatment selected by the guideline for that cancer at that stage? This is the result of the adoption of evidence-based medicine, the third pillar of the current cancer management system. According to the concept of evidence-based medicine, if a cancer treatment has been shown in a “reputable” clinical trial to be effective or better than a previously used treatment in a group of cancer patients, it then can be adopted for future use in other patients sharing similar tumor type and TNM staging. One does not need to understand or explain why such a treatment is effective or better. On the other hand, if a treatment has not been proven by “reputable” clinical trial to be better than existing treatment regimen in a group of patients, regardless whether it is reasonable and explainable by known mechanism, it should not be used on any patient. In other words, evidencebased medicine only recognizes “evidence”, not mechanism or reasoning, for clinical application. As results, the current cancer treatment guidelines are filled with standardized care plans that are not explainable by mechanism (why this treatment is better that the previously one?). One can only see what it is, but not why it is. Thus, one cannot deduce future direction of improvements from these standardized cares other than bluntly trying random combinations of previously existing treatments in hope to find one better. Now days, medical journals are filled with such boring and sometimes even seemingly unreasonable trials that often do not contain any intellectual challenge and stimulation. This is the current status of clinical research in cancer medicine controlled by the concept of evidence-based medicine.
What are Wrong with These Three Pillars?
The TNM staging system has come to today’s shape through improvement from initial assessment based on measurements of primary tumor only. Among the parameters, the number and location of metastasis are more significant towards prognosis. Even with a small primary tumor, so long as distant metastasis (M) exists, the case is designated as stage IV with grim outlook. Realization of the significance of metastasis over primary tumor represents a significant progress of the predictability of each cancer case, thus contributing to a better management [3]. Because of this realization, current management guidelines follow the designated staging to recommend treatments, especially when it comes to surgical treatments. Regardless of the easiness to be removed by surgery for a primary tumor, the presence of distant metastasis forbids surgical treatment in general. While this may actually avoid disastrous consequence of surgical treatment, it also shuts the door of clinical cure for many who present with a stage IV disease. But numerous clinical observations have indicated that not all stage IV cancer cases cannot be cured by surgery, some could be while others were not. For example, light burden primary lung cancer cases with single brain metastasis are designated stage IV disease, yet cancer surgeons have repeatedly tried to eradicate the disease by operating on some with good conditions, and some have indeed been saved by surgery on the primary tumor and brain metastasis [4-6]. The problem is: with some saved by surgery, others fared worse with shortened survival and there is no telling who may benefit and who may suffer from this treatment. After many years and multiple trials, the consensus now is that there is no definitive way to select stage IV patients for beneficial surgical resection, thus the safest way to approach this problem may be to eliminate surgical approach all together for all stage IV patients. On the other extreme, patients with early stage may also fare differently after primary tumor resection. Whereas majority of these patients remain clinically cured by primary cancer surgery, quite a few (10- 50%) do have recurrence and die as a result [7,8]. Same as to Stage IV disease, there is no telling who may be saved and who may not. Most intermittent staged cases (for example stage II-III) are even more difficult to accurately predict treatment consequences based on their TNM designation. In reality, TNM staging for each cancer case is more of a selection criterion for standardized care plans than predictive criteria for the prognoses under the selected treatments. Even this role of TNM staging has been seriously challenged by some of the most recently developed therapies. For example, suitable patients for the immune checkpoint inhibitor (ICI) therapy cannot be selected based on the TNM staging of a case. The response is also not related to TNM staging of a case [9,10]. Targeted therapy with small molecule TKI (threonine kinase inhibitor) drugs are also not selected based on the TNM staging of a case and the likelihood of responses to the therapy is not related to the TNM staging of that case, either. As results, other selection criteria, mostly molecular markers, have been identified for these therapies [11]. The purpose of these new molecular markers is to better understand each tumor so that a more effective therapy may be applied. In this regard, these markers are extensions to the TNM staging in that they help to place each cancer case to a specific starting point from which a specific treatment plan may be designed for that cancer case only. Some of these markers are cancer related genetic changes that reflect certain characteristics of that tumor. For example, mutations that drive the autonomous replication of a tumor [11], other markers are not directly related to cancer replication, but the likelihood of their genetic disorder. For example, the tumor mutational rate (TMB), the genetic instability (MSI-H), and other known mutations that may reflect genetic stability (dMMr) [9,10]. Like the TNM staging, all of these markers are about each tumor, what they have, what they look like. Yet, cancer growing in a patient have extensive interaction with its host. The biggest shortcoming of the TNM staging is lack of direct reflection of this relationship. For example, recent clinical observations have indicated strong and even decisive influence of host antitumor immunity on case outcome [12]. Inasmuch as cancer cases may have very different host antitumor responses among themselves due to varying genetic composition in each individual, the current TNM staging cannot fully reflect these differences, and thus cannot place a case at an accurate starting point from the immunological point of view. It needs to be pointed out that the TNM staging does reflect the host antitumor immunity indirectly. The contributory role of metastasis over primary cancer in the TNM staging has emphasized the role of host antitumor immunity because the presence and degree of metastasis is heavily influenced by host concomitant antitumor immunity. Nevertheless, no current clinical staging (not limited to TNM criteria) that helps to place each cancer case to a proper treatment plan has incorporate the status host immunity into consideration. This is also the root of the problem for the other two pillars.
Because of the limitation of TNM staging, patients who have quite different concomitant antitumor immunity status are placed into the “same” group and treated with the same plan. This practice, called standardized care, has always failed to yield similar responses to the same treatment, indicating that the patients placed under these “standard” treatments are indeed not same at all. But with the limitation of TNM staging, what are different in these patients are not recognized. Why standard treatments do not yield standard responses? Is this standardized care the best way to treat cancer patients? A more direct question is: With all clinical observations against presence of a unified patient group even with similar TNM staging, why they are placed into standardized but not individual care? One cannot fathom a reasonable answer. From logic point of view, it is clearly unreasonable to treat different patients with the same treatment while many other therapies are available for selection. An even more unreasonable approach is to place hundreds of thousands of patients to the same treatment regimen knowing that a majority of them may not benefit from such arrangement. There has to be a reason for this unreasonable practice. One possible explanation comes from historical practice of drug development. In order to sell a new drug, dug makers needs to convince regulatory agency that its drug is “effective” in a group of people. The drug maker as well as the regulatory agency would prefer this group of people to be similar, so that the efficacy may be more assessable. The newly tested drug needs also demonstrate that it is “as good as or even better” than current drug on the market, so a comparison group (also called the control group) has to be included into the trial. This trial may yield a “result” as Figure 1A illustrates. The interpretation of this result is straight forward in that drug A (the newly developed drug in this case) has seen 35% responses in its group, while drug B (the control drug) has shown 25% responses in its group. As long as both groups contain the “same” mix of patients (a prerequisition that can be satisfied as long as large number of patients are included in each group), one can conclude that drug A is better than drug B in terms of yielding more responders, thus benefits, in a group of patients. For the regulatory agency, this is good enough to approve the sale of drug A to the public. For the drug maker, it is good enough because they do not decide how is a cancer patient treated, they just provide one option by providing the drug. The clinical trial they performed is for marketing approval, not for best use of their drugs in a complicated individual case. It is up to the clinicians to figure out how to treat cancer patients with all available drugs and means. The clinical trial performed by the drug developers for regulatory approval is only a reference, not a mandate. Yet because the complicated nature of cancer, doctors look for accomplished clinical trials for reference or copy rather than tested out in patients by themselves. This practice eventually forms “consensus” by doctors to relay others to provide them with best use of newly developed drugs, thus the birth of guidelines put together by respectable academic clinicians. This is the birth of standardized care based on “evidence”. This way of clinical trial and data interpretation have been adopted by mainstream medicine in their own clinical research as that almost all clinical trials are set up with the same style used in drug development (Figure 1B). This is because to perform any alternative treatment regimen, even with the same drug, seems prohibitingly expansive and efforts consuming for most academic clinicians that the trial carried out by drug company often becomes the “standard” protocol even though its initial purpose was not the best use, but to beat current comparison. Further, the initial interpretation of the comparison trial data bears no logic that a drug or treatment with a better response rate in a clinical comparison trial should be applied on all patients in the real-world clinical setting. In a group of identical patients, it may be true that a wining treatment is also a better one. But we know that all patients even with same TNM designation are fundamentally different. In 100 mixed patients, 35 of them may be more suitable for drug A, while another 25 may be more suitable for drug B. Just because drug A has more responders than drug B does not mean that all patients should be subjected to drug A. The correct way is to place the 35 patients who are suitable for drug A to drug A treatment and the 25 patients who are suitable for drug B to drug B treatment. In this way, 60% of all patients will benefit from existing options (Figure 1B). Compared to this reasonable approach, the standardized care in the current form that forces all patients of the same TNM designation to a single treatment plan not only is unreasonable, but also harmful for a majority of patients.
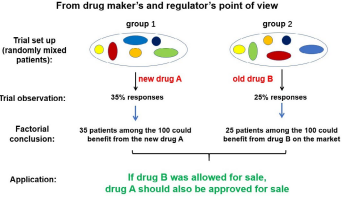
Figure 1A: The clinical trial set up, findings and interpretation/ application during drug development. The purpose of the trial is to answer whether the newly developed drug (A) can bring clinical benefits to more people than that of a previously approved drug (B). Even if the two groups contain different individuals (indicated by various colored balls), and drug A and B may have preferential recipients, so long as the numbers of patients in each group are large enough to have a similar mix, the finding from such a trial can answer this question, and can provide sufficient evidence for the regulatory agency to consider a commercial approval for drug A.
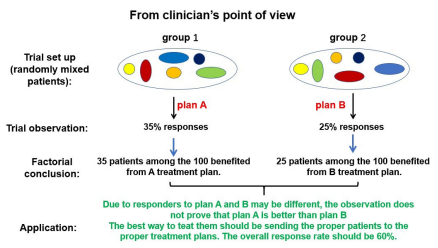
Figure 1B: The meaning of a similar clinical trial set up, findings and interpretation for selecting therapies for cancer patients. This is a typical trial set up similar to the drug approval trial in Figure 1A. If different individuals are included in each group to from a mix, the true meaning of the data can only be the response rate for each treatment plan, not that one plan is “superior” than another because different patients respond differently to different plans. It is wrong to force all patients in the group to accept only one plan based on the meaningless comparison.
The establishment of standardized care is based on the rationale that these patients are the same, thus should benefit on selected treatment plan that obtains the most response rate. The selection of this “optimal” plan is based on clinical trials that compare different drugs or combination of drugs or other treatments (for example radiation therapy). Once selected, it is presented to the medical field in the form of expert consensus called guidelines. Once published, every physician is supposed to follow the guideline-depicted treatment plans down to the exact details. This is called evidencebased medicine. Any attempt to challenge or change the guidelinedepicted treatment plans shall follow the same procedures and standards based on which the guidelines are established, regardless whether the depicted regimen or the proposed changes are reasonable. Nothing is wrong to base treatment plan on evidence. But it is wrong to base treatment plan only on evidence obtained in the previously adopted style and form from drug development (Figure 1A). It is also wrong to discourage attempt in the form of scientific reasoning. The power of science is not just the ability of fact-finding, but also the ability of logical thinking or reasoning and deduction. As discussed above, one can easily see the problem of recommending different patients to the same treatment plan that may only be beneficial to a portion (even not a major portion) of them. This is a logic argument that do not need clinical trials to prove (as it is already proven that not all “same” patients respond equally to the same treatments). Yet, the mainstream medicine does not allow such argument to interfere with their standardized care guidelines. Their argument is that they have clinical “evidence” to support their selection. Whoever does not agree with their selection, they need to show the evidence obtained with the clinical trials under the same “set up”, i.e., statistical comparison using groups of mixed patients. Findings from other way, for example from individualized treatment, are not considered “reputable or creditable” evidence, thus are ignored. The problem is this: cancer is an individualized disease due to individual differences in tumor structure and replication as well as in host immune responses to each tumor, The latter varies widely among cancer patients who even share the similar TNM designation. The correct and best way to manage such individual disease is through individualized care, but not unified standardized care. Thus theoretically, 100 patients may have 100 different treatment plans tailored specifically for them. Suppose such management has been accomplished, how can the results be compared to standardized care? Will mainstream medicine recognize such comparison as reputable or creditable evidence? Take a recent example of how to select patients for immune checkpoint inhibitor (ICI) therapy, many studies have selected various markers and compared the statistical significance in retrospective analysis. Some of these analyses have yielded markers that are marginal predictive for benefit, such as PD-L1, TMB, MSI-H, dMMr, etc. [9,10]. USFDA has also approved some of these markers in clinical selection of patients for ICI therapy. As we have established a depletion model to explain the various observations in ICI therapy [13], we have developed a set of criteria to distinguish each patient for their likelihood to benefit or suffer from ICI therapy. This method has very high accuracy so that we can accurately identify patients who should not be subjected to ICI therapy due to depletion of their concomitant antitumor immunity and subsequent tumor progression as results. As majority of randomly met patients do not fit for ICI therapy, this will save them from suffering due to wrong decision to give them ICI antibodies. We can also accurately select the few who may benefit from ICI therapy (about 20% of randomly met patients). But this kind of evidence is not accepted by mainstream medicine as it is not obtained with unified standardized treatment and group comparison. They would like to see clinical trials that follow the set ups they are familiar with, not any other way, even though our data has extremely high statistical significance that we must be right. Not only we are right by statistical analysis, but we also provided why we are right through scientific reasoning based on clinical observations that is commonly available to all who care to take a notice. Regardless, such analysis is not acceptable for publish in “reputable” medical journals. This is what is wrong with “evidence-based medicine” in the form as we know it.
What should be the Right Way to Treat Cancer?
Cancer is an individual disease due to unique host-tumor interaction. This interaction in the form of immune response is unique to that tumor because tumor antigens are presented by individualized HLA complex and recognized by individual TCR arrangements that is unique to that host. Since these interactions have the most influences on cancer development, responses to various treatments and death, differences in these interactions among different patients predispose them to different prognosis [12]. When treated by the same treatment, the differences in these interactions predispose patients to different responses, some see benefits while others suffer damages [14]. Thus, the reasonable approach to this individual variation is to individualize treatments according to the individualized interactions in each patient. Since the TNM staging does not fully reflect the individualized interactions between tumor and its host antitumor immunity, this approach of individualized treatment cannot be built on current form of TNM system. In order to establish an individualized cancer management system, we need to modify and extend the current TNM staging system, adding measurements reflecting the levels of antitumor immunity in each patient. As there is no established method to evaluate the interaction between tumor and host antitumor immunity, the first step would be to establish such a method of assessment. We have been looking into this aspect in the past few years and have explored some practical ways to evaluate the antitumor immunity in a host. One way is to look into tumor tissue for the signs of immune response. These include the number and distribution of T cells inside tumor tissue; the subtype of T cells (CD4/CD8), the functions of T cells in term of suppressing tumor replication and destroying tumor cells and structure. Figure 2 is an example of such analysis in a surgical sample of a cholangiocarcinoma patient. This patient had no symptoms while the tumor was discovered through routine physical checkup. Upon elevated tumor marker CA199, further imaging tests were carried out and a 6cmx5cm nodule was found at the location of common bile duct, supporting the presence of a cholangiocarcinoma. The tumor was surgically removed. Pathology report confirmed a cholangiocarcinoma with medium to low differentiation. A labeling-index of 70% for the tumor replication marker Ki-67 was mentioned in the report. Although no local or distant metastasis was identified, hospital provided a grim outlook based the poor prognosis for cholangiocarcinoma, the size of the primary tumor, presence of low differentiation structure and the high replication of the tumor. They believe that this case had very high risk of post-surgery recurrence, and once that took place this case became hopeless. As result of this assessment, adjuvant chemotherapy was prescribed. Patient and relatives turned to us for help since they could not accept the diagnosis and outlook. We examined the surgical sample and Figure 2 shows what we saw when the status of immunity was evaluated. The tumor tissue had two clear “structures”: majority part of the tissue had a solid structure, resembling lowly differentiated cancer (Figure 2A, lower panel, HE), while sporadic part of the tissue had the structure of a medium differentiated tumor resembling adenocarcinoma (Figure 2A, upper panel, HE). Tumor replication as reflected by Ki-67 labeling showed sporadic distributed single cell signals in the lowly differentiated area (Figure 2A, lower panel, Ki-67) while high activity in the adenocarcinoma structure (Figure 2A, upper panel, Ki-67). It was based on this high activity that the pathology report cited a 70% number. When T cells were stained, we saw uneven distribution pattern: very large number of T cells was accumulated in the solid area and surrounding adenocarcinoma structure (Figure 2A, upper and lower panel, CD3), while only a few can be seen at the adenocarcinoma structure (Figure 2A, upper panel, CD3). Majority of these T cells are of the CD8 subtype. Some show activated state as reflected by circled shape and CD3 staining (under high magnification). Since we know that T cells accumulated in tumor are tumor-specific and have antitumor activities, this uneven distribution of T cells between the two different structures may reflected an antitumor process in which the normal tumor structure of cholangiocarcinoma represented by the medium differentiated structure was destroyed by antitumor T cells to form the solid area or lowly differentiated structure. In fact, we could find some area in the sample where the process of immune attack and destroying tumor structure was underway (Figure 2B). With an immune response, not just T cells will accumulate the site of antigen, so are other immune cells such as macrophages, dendritic cells, neutrophiles. The presence of large number of these cells fills the stromal/mesenchymal space thus collapsing any previous structure to form cell-pact concrete structure. With some of these cells also carry out scarring process, this solidified space may eventually turn into fibrosis after withdraw of immune cells, which is also present in this sample (not shown). This observation of the presence of a strong antitumor immunity is consistent with the lack of metastasis as concomitant antitumor immunity has the ability to eradicate small established or newly established metastasis even if it cannot eradicate the larger primary tumor. It is also consistent with the lack of symptoms in this case as the most effective antitumor responses often do not cause symptoms (our unpublished observations). From the small residual portion of adenocarcinoma in the entire removed sample, one could construe that the antitumor immunity was at the upper hand at the time of diagnosis and surgery and this case may actually self-heal without notice if not discovered by a checkup not related to cancer. Based on these observation and interpretation, we concluded that with the removal of all visible tumor nodule (judged by the return of CA199 to low level below normal range), the residual antitumor immunity should turn into a strong immune memory that will protect this host from establishing any recurrence at least in 5 years to reach a clinical cure. Therefore, it is not necessary to accept post-surgery adjuvant chemotherapy. Patients and relatives accepted our interpretations and recommendation, went on without chemotherapy till now, recurrence-free more than four years after surgery. This example demonstrated that by looking tumor from an additional dimension over the traditional TNM staging, one can see each cancer case much better without relying on statistics to predict outcome. This additional dimension over the traditional two-dimensional TNM is host antitumor immunity. Just like in the TNM staging, status of metastasis dominates over primary tumor in staging assignment, status of the host immunity dominates over tumor distribution pattern in determining prognosis in this threedimensional staging system (Figure 3A). In this staging system, some of the early staged cases are not true in prognosis due to low concomitant immunity, whereas some of the late staged cases are not true late because they have a high concomitant antitumor immunity (Figure 3B). This newly established staging system still needs more fine tuning, but the general frame-working is correct and has been supported by clinical observations in our hands in the past 6 years since its creation.
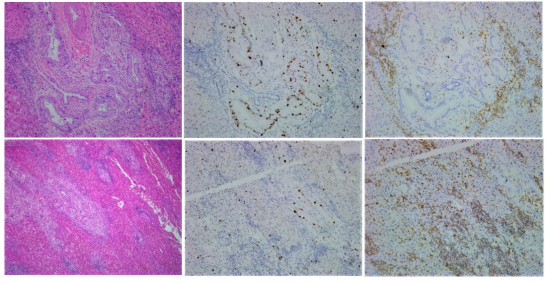
Figure 2A: Observations from the surgical sample from a cholangiocarcinoma patient stained for structure (left side column), tumor replication (Ki-67, middle side column) and presence of T cells (CD3, right side column). The tumor tissue showed two types of structure/differentiation: one is the typical adenocarcinoma with medium differentiated structure (upper panel, HE); the other is a solid/ low differentiated area, occupy >70% of the tissue (lower panel, HE). Tumor replication is high in the adenocarcinoma structure (upper panel, Ki-67), while only sporadic signals can be seen in the solid area (lower panel, Ki-67). In contrast, T cell distribution is massive in the solid area with patches of T cells accumulate together (lower panel, CD3). Most of these T cells are of the CD8 subtype, some show activated states (circled staining pattern). On the other hand, only few T cells are present inside the adenocarcinoma structure (upper panel, CD3).
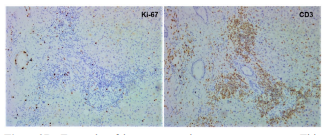
Figure 2B: Example of immune attack on tumor structure. This is one part of the surgical sample from the patient in Figure 2A, showing that the adenocarcinoma tumor structure collapses upon invasion by large number of T cells (and other immune accessary cells). It is also noticeable that tumor replication in the remaining structure surrounded by T cells is almost shut down, indicating that T cells have the antitumor functions of both suppressing tumor replication and destroying tumor structure. From this observation, one can deduce that the solid area in most of the residual tumor tissue at the time of surgery (Figure2A, lower panel) is formed by immune attack.
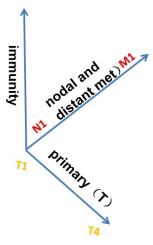
Figure 3A: Illustration of the three dimensions for cancer staging. An additional dimension of concomitant antitumor immunity is added on top of the current TNM system to provide a more accurate positioning of each cancer case. The application of this staging system and its relationship with the current TNM staging is illustrated in Figure 3B.
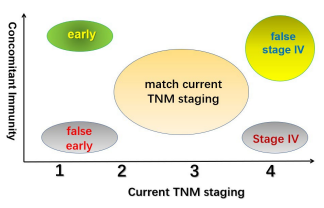
Figure 3B: The relationship between the three-dimensional cancer staging with addition of antitumor immunity as an independent dimension on top of the current TNM staging. Some of the “early” staged cases by the current TNM may no have the good prognosis associated with early staged cancer due to lack of concomitant immunity, thus are designated as “false early”. Similarly, some of the late staged (stage IV) cases may not have the poor prognosis associated with Stage IV cancer on the TNM staging system due to presence of a strong antitumor immunity, thus are designated “false stage IV” on the new staging system. The new staging system has many overlaps with the current TNM staging in the medium staged cases due to not so strong antitumor immunity. Overall, the status of antitumor immunity in this staging system is dominant in that true early and late staged cases are determined by the presence and absence of antitumor immunity.
Cancer metastasis is the process and event that kills the host eventually. This metastasis-forming ability is the characteristic of each individual tumor. This includes the ability to disseminate from the primary tumor, to replicate autonomously and to establish angiogenesis [15]. Many tumor cells have these abilities or else they would not be cancer. But some may not. In many cases, we can deduce or detect the presence of tumor cells that do not have the ability to replicate autonomously [16]. These tumor cells depend on host factors to replicate. These host factors are often associated with local inflammation incited by other autonomously replicating cancer cells at the same location [17,18]. To recognize the presence of these two populations of replicating tumor cell and their interdependent relationship is a critical step towards better prediction of several outcomes in clinical management of cancer. One of them is the potential response pattern to TKI drugs targeted to the autonomously replicating population [19]. The other is the metastasis formation at distant sites [16]. The current TNM staging system, although emphasizing on distribution of metastasis, does not distinguish the mechanism behind the lack of distant metastasis. It cannot answer whether a case that lacks distant cancer at the diagnosis will remain so or metastasis may form at any time in the near future. With the establishment of the mode of tumor replication, combined to the status of host antitumor immunity, one can answer why a cancer case at diagnosis lacks distant metastasis and predict whether new metastasis is likely to form in the near future. This is critical in some cases where lack of clear view caused over treatment of a non-autonomously replicating tumor leading to generation of autonomous replicating mutations and formation of true metastasis [16]. It needs to be pointed out that this look into the mode of tumor replication is broader than the tumor mutational analysis currently in clinical application in that the current genetic analysis focus on finding the so-called driver gene mutation to see whether certain targeted drugs may be used [11]. The analysis of the mode of tumor replication proposed by us includes genetic search for the driver gene in an autonomously replicating tumor not just for the purpose of targeted therapy, although it is a much more accurate way to predict the outcome if therapy is applied [19]; but also for prediction of whether a population of non-autonomously replicating tumor cell exists and the likelihood of distant metastasis formation [16]. It also provides the assignment of sensitive tumor makers to the replication activity of each population, thus effectively tracing responses from various treatments (our unpublished observations). In summary, this analysis of the mode of tumor replication represents a broad area of tumor biology previously ignored by the TNM staging. Its establishment, together with the above-mentioned host antitumor immunity, forms the two additional dimensions in cancer staging. We have applied these combined analyses in the clinical setting for every cancer case we deal with. After more than six years in practice, predictions made on the early cases have come in and they turned out to be over 70% accurate (results to be reported elsewhere). With time and recent progress, we expect further increase of accuracy for the predicted outcome to reach over 90%.
The accurate assessment of a cancer case is the pre-requisite for accurate prediction of its prognosis. Based on this prediction, we can then answer whether a case can be clinically cured with the current available methods, and if not, how long the patient may survive. This is critical for the selection of correct pathways towards maximal survival. This entire process is evidence-based because the observations on tumor replication and antitumor immunity are true. Subsequent analysis and selection of therapy are based on the observation. It is also individualized in that each cancer patient is treated according to his specific situation, not just his TNM staging, but also the mode of tumor replication and the status of his antitumor immunity. This entire process does not rely on how other patients with similar TNM staging are treated, thus it is an individual but not a standardized care. Then, how can we know this individualized care is better than the current standardized care? Bases on the current practice, it has to be shown in a head-to-head comparison with the current standardized care in which patients with the same TNM staging are randomized and divided into two groups. One group of them receives the standardized care and the other receives individualized care. While such a comparison could prove the superiority of individual care, it is unethical for anyone who understand the principle and practice of individualized care to deliberately carry out such a comparison knowing that any patient sent to the control group for standardized care will face much less effective therapy, some may even be harmed. For investigators not sure about the superiority, such comparison may be not unethical (since they do not deliberately harm the patients), but they would not be able to carry out individualized medicine because they do not know how to do it. This is a dilemma, but not impossible. One needs to have an open mind when looking at the evidence. Decades ago, some doctors in Japan developed the D2 vs D1 resection for surgical treatment on stomach cancer, a cancer with much higher incidence in East Asia than in the Western countries. They saw clear benefit of the extended surgical approach in almost every patient in that the post-surgery recurrence rate in these patients dropped precipitously. They began to report such an observation in medical conferences in Japan and more surgeons responded with enthusiasm and acceptance. Yet this practice was heavily criticized by the peers in the Western countries for lack of “evidence”. Ignoring the fact that compared to the traditional D1 resection approach, very high portion of patients receiving D2 resection survived without recurrence, they insist for a headto-head comparison between the D1 and D2 surgery in a single clinical trial. Doctors in Japan refused to carry out such a trial knowing that any patient randomized to receive D1 surgery faces much higher risk of recurrence and death. The Western countries finally did one by themselves and reported an unfavorable finding in the top medical journal, criticizing the extended surgery caused more surgery-related death and no clear benefit. But Japanese doctors ignored such a ridiculous finding and went on with more and more D2 resection performed because they did not see much higher surgery-related death in their hands. This D2 resection was also accepted by most East Asia surgeons for the benefit was clear, so clear that there was no need for a head-to-head clinical trial to convince anyone who are familiar with such surgery. Eventually, the Western investigator gave in based on the subsequent long-term recurrence-free observation in their trial [20]. Was it absolutely necessary to carry out such a clinical trial? For some who were used to the current drug development-related style of clinical trial, it was necessary to change their minds. But for many others who base their judgement on common sense and experiences, it was not necessary. The proof of individualized care in cancer management may require the same open-mindedness. Even by the abstracted way of logic comparison, one sees the superiority of individual over standardized care, because at the worst, all individual care cases end up selecting the treatment plan used in standardized care. That proves that there is no need to individualize, not because it is harmful, but a waste of resource. On the other hand, if one sees clear superiority of individualized care in patients with various cancer and TNM staging compared to historical date obtained in decades of standardized care, one can easily deduce the outcome of a head-to-head comparison trial. Unfortunately, we have seen so many experts with stubborn mind in recent years when we tried to explain what is an individualized care for cancer and why it is superior. For these people, any challenge to the current standardized care and guidelines, a head-to-head comparison trial is a must, regardless when the difference between the two is large and clear. The problem is that through decades of education and the bombardment of medical journal publications with statistical results from various clinical trials in which individual patient is only a number, doctors are conditioned to accept the so-called “evidence-based medicine”. Since the majority publication of clinical observations only describe what it is but not why it is, doctors lost the ability to reasoning and looking for answers not just data in their medical research. The reasoning analysis as we practice in individualized care is so unfamiliar with them that they simply ask whether we can provide a finding from a head-to-head comparison in a style they are familiar with before they agree to look into this issue. For most cancer doctors, understand antitumor immunity is not something they can easily pick up, less to say that they can apply that in their daily practice. This constitutes a major obstacle in training doctors to individualize cancer care based on the comprehensive staging system including mode of tumor replication and status of antitumor immunity. But that should not be the reason why cancer is not treated by better medicine, in this case, individualized care.
In contrast to the group data-based clinical research without emphasizing on mechanism, pre-clinical research based on individual animal tumor model has long focused on unique observation and the mechanism behind the observation. A misconception is that these findings represent a common finding applicable to all similar cancers with similar TNM staging. The “translation” of these pre-clinical findings, especially findings from tumor immunology study, has failed to show the same benefits in cancer patients in almost all cases. The real reason is not the differences between mouse and human (in fact, their immune systems and tumor behavior are very close), but the mismatch to apply something found in one individual to an entire group of mixed individuals who have huge differences in status of their antitumor immunity. If the treatment is applied to one cancer patient with similar characteristics (for example, sufficient antitumor immunity), the translation should work just fine. Take the example of adoptive transfer therapy of tumor-infiltratinglymphocytes (TIL), it was developed in individual animal models [21] and when applied to cancer patients indiscriminately, it had failed in most patients, because TILs from most random patients either failed to expand in vitro or expanded population were unrelated T cells without specificity for their tumor. Only those who share the same strong concomitant antitumor immunity like the animal tumor model where TIL therapy was initially developed may provide sufficient TIL for in vitro expansion. And in such patients, the chance of success was much higher (Dr. S. Rosenberg, Surgery Brach, NCI, personal communication). There is nothing wrong to move the successful research from individual animal models to human so long as the translation is targeted to proper cancer patients. On the other hand, it is also productive to focus on individual cancer patients for clues of why certain therapies work extremely well in them. In this regard, we have quite a few successful cases in the past 6 years. The more recent example is to find out the mechanism behind the immune checkpoint inhibitor (ICI) therapy. Many studies try to look for patient selection criteria not by studying the mechanism, but by screening massive patient data for clue, and some did yield predictive markers such as tumor expression of PD-L1, tumor mutational burden (TMB), micro satellite instability-high (MSI-H), dMMr defect and several others [22,23]. It is fine to look for clues this way, but once something stands out, one need to explain why it is pointing to the patients who may benefit. Without the latter process, we often get lost in the massiveness of data and the way we mine these data (analyses). On the other hand, common sense tells us that the essential factor(s) that make ICI therapy work should stand out much clear in all patients who responded to ICI therapy extremely well, so one does not need many patients to look for such factors, only the few with the best responses should be enough. Looking at these cases carefully and deeply, one can definitely find what he is look for. Indeed, we have done so and we have found what we are looking for by just studying the few super-responders in our hands. One common feature of all these super-responders is the pattern of T cell infiltration: evenly mixed with tumor cells in a low differentiated structure. No consistency on the number of T cell or the subtype of T cell was seen in these cases, thus the level of concomitant immunity may not be critical for responding well to ICI therapy. Different to the mainstream research that only cares finding what it is but hot why is, we ask why this pattern of T cell infiltration (we call it mixed mode of T cell infiltration) is critical for responding to ICI therapy. Our reasoning based on several other clinical observations, has produced a new working model of ICI therapy in which this pattern of T cell infiltration protects deeply infiltrated T cells from antibody-mediated depletion and subsequent activation of these T cells through homeostasis-driven expansion due to imbalanced T cell concentration caused by depletion of T cells in the parenchymal/stromal space [13]. So far, this newly proposed model can explain all clinical perplexing observations that the previous blocking model cannot explain. From this new model, we went back to all previous cases of ICI therapy and found out why some of them (a large portion of nearly 40%) had suffered from the therapy, indicating that our model is likely correct. We also checked extensively in previously published studies in which T cell infiltration patterns of responders can be found, and in every study we saw, the responders all match our selection criteria [24- 27]. We then tested the accuracy of prediction by our new model in prospective patient population. So far, all 8 cases that we deem high responders beforehand and took the ICI therapy have responded well, while all continued cases from outside in which ICI therapy was bluntly used without good justification but showed responses were found to match the responder profile by our model. On the other hand, we have recommended against the use of ICI therapy in many more cases, because most randomly selected patients do not meet the responder criteria by out model. We cannot prove that we have avoided harm to these patients by standing against the use of ICI therapy, but occasional cases not following our suggestion in which ICI therapy was used all showed hyper-progression of tumor and shortened survival (our unpublished observations). This is an example to illustrate that a useful clinical study can be based on a few cases as long as the finding has no exception, explains why it is and then can be tested in other cases. This is what has been done in pre-clinical studies for many years by the mainstream research community. We don’t see why it is not suitable for human study. This example shows this type of analysis is very proper and powerful to generate valuable results. We have even done a study that generated significant findings in a single case [19] that is applicable to many. The important factor that secures a success in a research is to ask questions from a unique observation till one gets a reasonable answer. This is evidence-based medicine, but this is also reasoning-based medicine.
In challenging to the traditional three-pillar principles in cancer management, the newly proposed cancer management system has extended cancer staging, emphasized on individualized care and proposed clinical studies that learn from the success of pre-clinical research. Without these changes, the current cancer management system with its deeply rooted limitations as discussed here cannot create the necessary break-through and win “the war on cancer”. If our own experiences tell us anything, it is the unique observation-based deep digging on the mechanism that is the direction of clinical research. Medicine without full reasoning will always remain as a trade of art, it will be a branch of science when logic and reasoning are used in data analysis, not just statistics. Using this approach, we have resolved many perplexing clinical observations and revealed several important mechanisms behind these observations such as the working of concomitant antitumor immunity in traditional cancer therapy [14]; the model of postsurgery cancer recurrence [28]; the hidden non-autonomous tumor replication and its connection to autonomous replication through inflammation [16]; mechanism of targeted therapy with TKI drugs [29] and its relationship to antitumor immunity in clinical use to avert drug resistance (to be published); mechanism of the abscopal effect by radiation therapy (to be published); mechanisms of ICI therapy and its beneficial as well as harmful effects [13]. These fruitful research findings have covered almost every important area of cancer management ranging from early to late and to terminal stages of cancer. And importantly, all of these achievements have been based on individual cases and accomplished without government or private funding. If we, a small group of people with limited resource, can accomplish such impressive research in seven years, imagine the current army of cancer research with unlimited resource can do if they are truly mobilized and go on the right way? To mobilize them, the first thing is to abandon or modify the limitations brought onto them by the three pillars of current cancer management. It is for this purpose that we present this writing.
References
1. Brierley J (2006) The evolving TNM cancer staging system: an essential component of cancer care. CMAJ 174(2): 155- 156.
2. Greene FL, Sobin LH (2008) The staging of cancer: a retrospective and prospective appraisal. CA Cancer J Clin 58(3): 180-190.
3. Burke HB (2004) Outcome prediction and the future of the TNM staging system. J Natl Cancer Inst 96(19):1408-1409.
4. Hankins JR, Miller JE, Salcman M, Ferraro F, Green DC, et al. (1988) Surgical management of lung cancer with solitary cerebral metastasis. Ann Thorac Surg 46(1):24-28.
5. Billing PS, Miller DL, Allen MS, Deschamps C, Trastek VF, et al. (2001) Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 122(3):548-553.
6. Lo CK, Yu CH, Ma CC, Ko KM, Leung SC (2010) Surgical management of primary non-small-cell carcinoma of lung with synchronous solitary brain metastasis: local experience. Hong Kong Med J 16(3):186-191.
7. Boyd JA, Hubbs JL, Kim DW, Hollis D, Marks LB, et al. (2010) Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol 5(2):211-214. 8. Hung, J.J., et al., Time trends of overall survival and survival after recurrence in completely resected stage I non-small cell lung cancer. J Thorac Oncol, 2012. 7(2): p. 397-405.
9. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, et al. (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349):409-413.
10. Darvin P, Toor SM, Sasidharan Nair V, Elkord E (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(12):1-11.
11. Chirieac LR, Dacic S (2010) Targeted Therapies in Lung Cancer. Surg Pathol Clin 3(1):71-82.
12. Hiam-Galvez KJ, Allen BM, Spitzer MH (2021) Systemic immunity in cancer. Nat Rev Cancer 21(6):345-359.
13. Tsung K, Zhanghui ZX, Tanlun Research Participants Group (2020) The Blocking vs Depletion Model of Immune Checkpoint Inhibitor Therapy for Cancer. J Cancer Res Rev Rep 2022. 4(2):4.
14. Guo H, Tsung K (2017) Tumor reductive therapies and antitumor immunity. Oncotarget 8(33):55736-55749.
15. Holmgren L, O’Reilly MS, Folkman J (1995) Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1(2):149-153.
16. Tsung K, Zhang Xu, Hui Zhang (2020) Existence and Significance of Non-Autonomous Cancer Replication for Clinical Management of Cancer: Observations and Hints from Ovarian Cancer Cases. J Pathol Res Rev Rep 2(2):1-8.
17. Saxon JA, Sherrill TP, Polosukhin VV, Sai J, Zaynagetdinov R, et al. (2016) Epithelial NF-kappaB signaling promotes EGFRdriven lung carcinogenesis via macrophage recruitment. Oncoimmunol 5(6):e1168549.
18. Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, et al. (2009) Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462(7269):104-107.
19. Tsung K, Zhang Xu, Zhao Jinming (2019) The Presence of Autonomous and Non-Autonomous Replication in Cancer and their Connection through Inflammation: Lessons from one Lung Cancer Case. Immunome Res 15(171):1-7.
20. Schmidt B, Yoon SS (2013) D1 versus D2 lymphadenectomy for gastric cancer. J Surg Oncol 107(3):259-264.
21. Rosenberg SA, Spiess P, Lafreniere R (1986) A new approach to the adoptive immunotherapy of cancer with tumorinfiltrating lymphocytes. Science 233(4770):1318-1321.
22. Shum B, Larkin J, Turajlic S (2022) Predictive biomarkers for response to immune checkpoint inhibition. Semin Cancer Biol 79:4-17.
23. Bai R, Lv Z, Xu D, Cui J (2020) Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 8:34.
24. Julie E. Stein, Evan J. Lipson, Tricia R. Cottrell, Patrick M, et al. (2020) Pan-Tumor Pathologic Scoring of Response to PD- (L)1 Blockade. Clin Cancer Res 26(3):545-551.
25. Tetzlaff MT, Messina JL, Stein JE, Xu X, Amaria RN, et al. (2018) Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol 29(8):1861-1868.
26. Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, et al. (2012) Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 7(5):825-832.
27. Zhang W, Yan C, Zhang T, Chen X, Dong J, et al. (2021) Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study. Oncoimmunol 10(1):1971418.
28. Chen X, Wang Q, Tsung K (2020) Antitumor Immunity as Determining Factor for Prevention of Post-Surgery Cancer Recurrence. J Clin Expt Immunol 5(1):1-11.
29. Tsung K, Zhang X, Zhag H, Zhao J (2020) Why TKI Therapy Targeting Minority Population of Tumor Cells Could Achieve Complete Control of Entire Tumor: The Self-Driven and NonAutonomous Replication in Cancer and Their Connection through Inflammation. J Clin Rev Case Rep 5(3):121.



