Research Article - (2024) Volume 1, Issue 2
Study of Biological CO2 Sequestration on Microalgae Species
2Gujarat Energy Research and Management Institute, India
Received Date: Jun 10, 2024 / Accepted Date: Jul 25, 2024 / Published Date: Aug 12, 2024
Copyright: ©©2024 Chetana Roat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Ansari, H., Hasan, S. Z., Kothari, F., Patel, T., Roat, C. (2024). Study of Biological CO2 Sequestration on Microalgae Species. Curr Res Env Sci Eco Letters, 1(2), 01-06.
Abstract
Microalgae represent efficient photosynthetic microorganisms with the potential to serve as natural air purifiers by generating oxygen and reducing CO2 levels. They utilize dissolved carbonate and bicarbonate from CO2 in the culture medium to support their growth. As they proliferate, the pH of the medium increases, creating favorable conditions for microalgae growth. This study focuses on characterizing microalgae for their efficiency in CO2 fixation and biomass production within a closed system. Optical density, pH, and dissolved CO2 levels were measured. Optimization of extraction methods for pigment and lipid production was conducted. The Bligh and Dyer method was employed for lipid extraction, while 99% Methanol was utilized for chlorophyll extraction. The study aimed to not only understand the carbon sequestration potential of microalgae but also develop practical strategies for maximizing their biomass and valuable product yields. In this study, the biomass productivity was obtained 80 mg/L for Chlorella sp. and 200 mg/L for Oscillatoria sp. The lipids, proteins, and polysaccharides present in microalgae biomass possess significant application potential, serving as valuable raw materials in industries such as biofuel production, biochemical manufacturing, food processing, pharmaceuticals, and various other sectors.
Introduction
Microalgae are microscopic, photosynthetic organisms found worldwide in water and soil. Microalgae may have different type of cell organization: unicellular, colonial and filamentous. Microalgae may be prokaryotic (no nucleus) or eukaryotic (with nucleus and other organelles). They contain important structures like chloroplasts for photosynthesis and nuclei for genetic information. They may have spores for reproduction and flagella for movement [1]. Microalgae require specific environmental conditions (light, temperature, etc.) for optimal growth and carbon capture. The ideal light intensity for most microalgae is between 26 and 400 µmol photons m−2 s−1 [2]. The impact of optimal temperature varies significantly depending on the species of microalgae. Maintaining a pH level between 6.5 and 8 is often recommended for successful microalgae cultivation [3]. Carbon, nitrogen, and phosphorus are vital building blocks for microalgae cells and biomass growth. Balancing the carbon - nitrogen ratio is crucial for optimal carbon fixation, biomass production, and valuable component generation. Increasing the concentration of phosphorus is beneficial to microalgae development and lipid accumulation. Microalgae use phosphorus for energy production (ATP) and cell wall formation [2]. Higher levels of CO2 were found to have a positive impact on the carbon metabolism of microalgae [3]. Different microalgae species have varying tolerances to CO2 concentration. Either a very high or very low CO2 concentration reduces the CO2 fixation efficiency and the biomass yield [2].
Oscillatoria sp., a type of filamentous cyanobacteria commonly called Blue-green algae, displays a filamentous structure composed of chains of cylindrical cells aligned sequentially. These organisms are frequently sighted on the surface of their environment. Chlorella sp., a species of unicellular green algae, typically exhibits a round shape with a diameter typically falling between 2 to 10 micrometers. These algae are commonly found in diverse aquatic habitats like freshwater ponds, lakes, and rivers. Carbon enters the atmosphere through natural and human processes, including organic matter breakdown, breathing, and combustion of plant materials and fossil fuels. Microalgae cells are primarily carbon-rich, accounting for half their dry weight. Microalgae, through photosynthesis, can effectively combat climate change by absorbing and storing carbon dioxide from the environment [4]. Microalgae absorb 10-50 times more due to faster growth, higher photosynthetic rate, and adaptability [2]. In CO2 sequestration, Calvin Benson cycle play a major role. Microalgae can absorb carbon dioxide from soluble carbonates, increasing pH and reducing nutrients in wastewater effluents [5]. They can absorb bicarbonate (HCO3-) and grow without competing with freshwater resources, reducing nutrients [6].
Microalgae biomass, rich in lipids, proteins, and polysaccharides, has high application value in various sectors like biofuel, biochemicals, food, and pharmaceuticals [2]. Its photosynthesis and carbon sequestration efficiency are influenced by species and environmental factors, making it a carbon-neutral single cell bio-factory. Proteins, carbohydrates, and lipids are extracted from algal biomass for animal feed and biofuel [5]. Microalgae can store 30-70% of their dry weight in lipids, making them Suitable for biodiesel production through transesterification. Carbon sequestration offers potential for hydrogen and electricity from fossil fuels, with power facilities in developing nations easily building COâ?? sequestration technology. Astaxanthin, which is derived from microalgae, a potent antioxidant, is used in cosmetics, red food coloring, and as a red food coloring. It promotes healthy fetal development, prevents cardiovascular disease, and improves memory function. Characterizing microalgae for their COâ?? fixation and biomass production efficiency within a closed system is a less expensive technique than building a photobioreactor.
Material and Method
Collection and Observation of Mix Microalgal Culture
Mix culture sample were collected from Algal Culture lab of Gujarat Energy Research and Management Institute, Gandhinagar, Gujarat. Observe it into the microscope under 100x. Observe the microalgal cells and their cell structure.
Isolation of Microalgae
The agar plating technique was used to isolate the microalgae, and the plates were incubated in a seed germinator until algal growth was detected. The isolates were purified by streak plating, and individual colonies were diluted in BG 11 medium. Observe the pure microalgal culture under a microscope at 100x magnification for identification. Selected two pure cultures inoculated into the BG 11 medium for further growth. The subculture method was utilized to promote growth.
Inoculation of Algae
Chlorella sp. and Oscillatoria sp. algal species were selected for the experiment. Inoculate 30% Chlorella sp. and Oscillatoria sp. algae culture into the BG 11 media. Take an O.D. at 750 nm for measuring initial growth. Directly purge COâ?? into both culture media for 2 to 3 seconds. Measure the pH of the medium and calculate the dissolved carbonate and bicarbonate. Incubate it in the seed germinator at 37°C. Measure the pH, O.D., and calculate the dissolved carbonate and bicarbonate every day. The titration procedure is given below:
Take a 10 ml sample and add 2 to 3 drops of phenolphthalein indicator. With the addition of the indicator, the sample color turns light pink. Titrate it with 0.01 N Hâ??SOâ??. At the end point of the titration, the pink color turns colorless. Note the burette reading as Y. Add 2 to 3 drops of methyl orange to that colorless solution, after which the color changes from colorless to yellow, and then titrate it with 0.01 N Hâ??SOâ??. The color changes from yellow to rose red. Note the burette reading as Z. And then calculate the carbonate and bicarbonate using the formula which is given below.
Lipid Extraction
Lipid was extracted using Bligh and Dyer method from the dried microalgal biomass. The dried biomass mixes with Chloroform: Methanol (1:2 v/v). After some time sonicate the sample using Ultra sonication. After sonication centrifuge for 5 min at 3000 rpm and oven dried and measured gravimetrically [8].
Chlorophyll Extraction
2 ml of culture was taken in a centrifuge tube and centrifuged at 5000 rpm for the duration of 10 min. The supernatant was discarded and 2 ml of 99% methanol was added into the pellet, mixed well and incubated for 24 hours. The following formulas have been used to determine the total pigment.
Chlorophyll a (µg/ml) = 16.72A665.2-9.16A652.4
Chlorophyll b (µg/ml) = 34.09A652.4 15.28A665.2
Carotenoids = (1000A470-1.63Chla -104.9Chl b)/221
Results and Discussion
Identification of Microalgal Strain
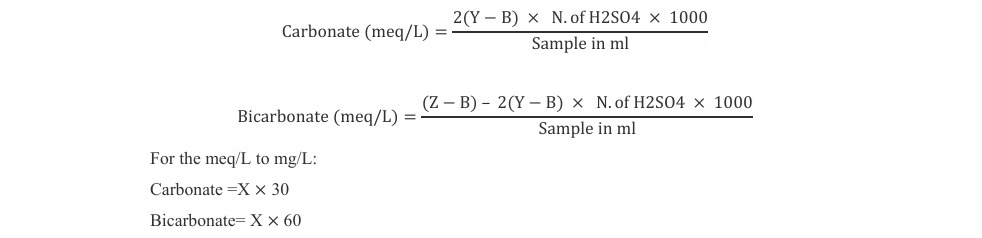
Isolated and selected strains are observed into the microscope under 100x. Chlorella sp. and Oscillatoria sp. are observed Curr Res Env Sci Eco Letters, 2024 In current study, Oscillatoria sp. and Chlorella sp. are growing well during a period of five to six days. The biomass productivity was calculated as 80 mg/L for Chlorella sp. and 200 mg/L for Oscillatoria sp. M S Mahajan et al. demonstrated that the lag phase of C. pyrenoids NCIM 2738 and S. platensis NCIM 5143 microalgae, with an average growth rate of zero, is characterized by little to no growth during the first six days of cultivation [8]. Furthermore, an increase in the linear pattern was noticed in the growth of microalgae. Maximum growth was seen on the 13th day of cultivation for these microalgae with 415 and 440 mg/L DCW in addition to 31.90 and 33.80 mg/L/day biomass productivity of C. pyrenoids NCIM 2738 and S. platensis NCIM 5143, respectively, as the incubation period increased. Bojan Tumbaric et al. demonstrated that the growth curves of C. vulgaris were measured by OD750 and then calibrated against the final DW. For the [C+] treatments, the final DW values were 0.34 ± 0.02 g L−1,respectively [4].
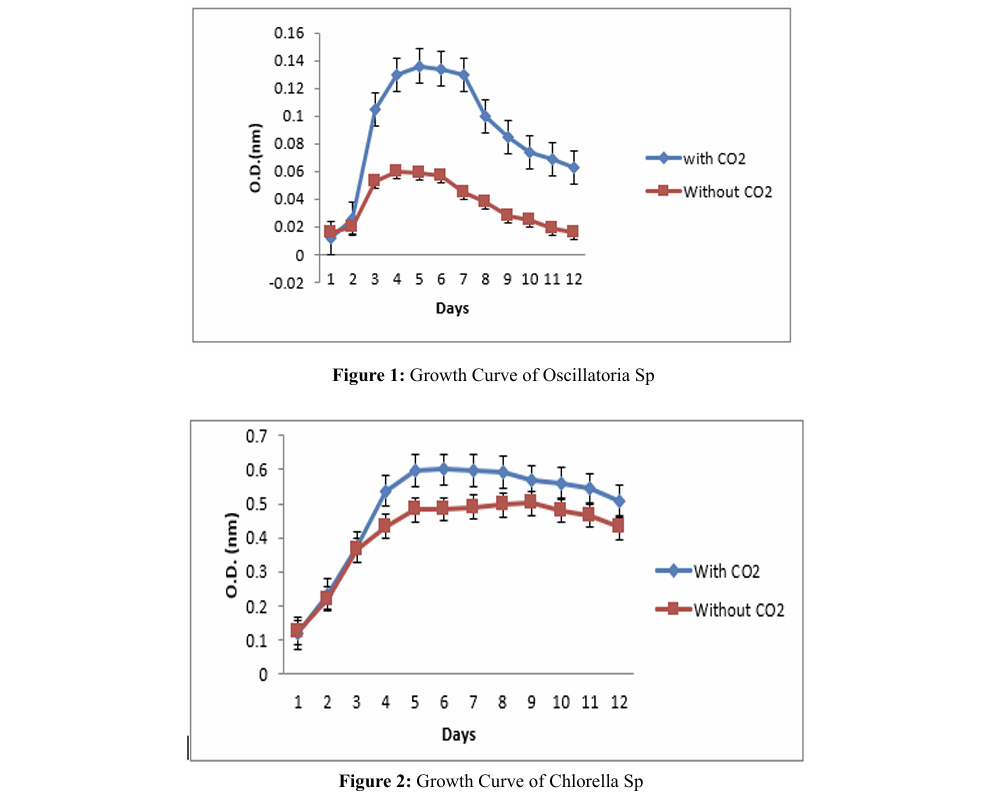
Effect of PH on Growth
The study demonstrates the growth of Chlorella sp. and Oscillatoria sp. for a period of 12 days with a pH range between 6.00 and 11.00, where as in the growth of C. pyrenoids NCIM 2738 and S. platensis NCIM 5143 for a period of 13 days with a pH range between 5.00 and 8.00 [8]. Chenba zhu et al demonstrated that S. platensis growth in medium with 300 mmol L NaHCO, and bubbled with air, with initial pH values of 10.0, 10.5 and 11.0. Overall, the final biomass concentration decreased with an increase in the initial pH, and a maximum biomass concentration was achieved at an initial pH of 10.0 [10].
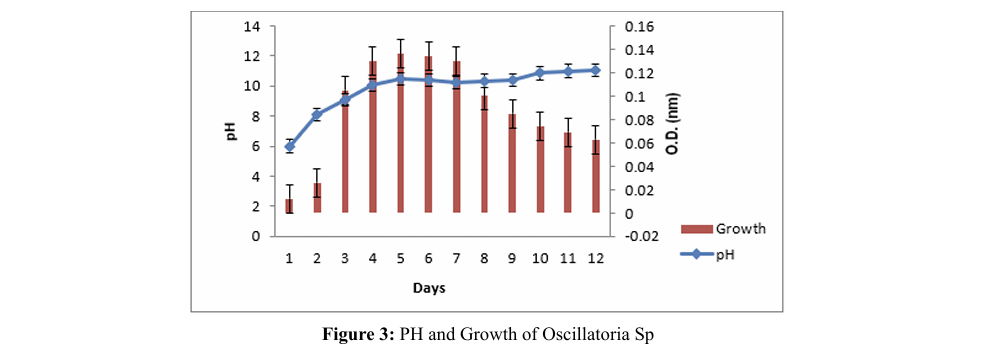
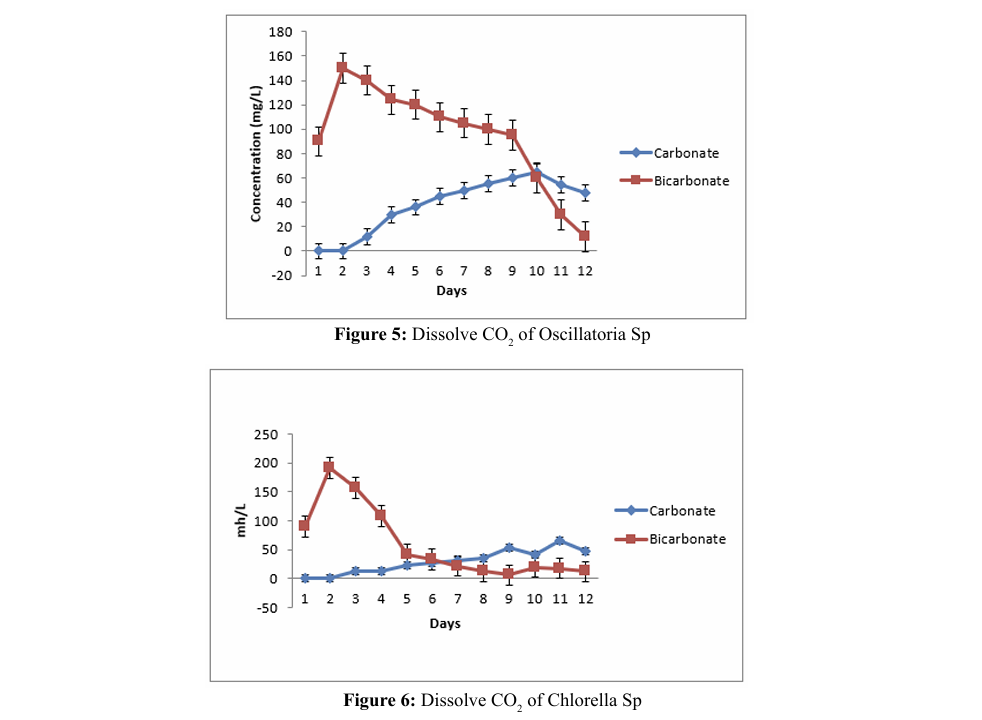
Dissolved Carbonate and Bicarbonate
Initially, CO2 reacts with water to generate carbonic acid, which then undergoes dissociation into bicarbonate ions (HCO3-) 3.4. Dissolved Carbonate and Bicarbonate and hydrogen ion (H+). Subsequently, the bicarbonate ions can further dissociate into either carbon dioxide (CO2 ) or carbonate (CO3-2), contingent upon the pH level of the medium. The concentration of dissolved carbonate and bicarbonate depends on both the CO2 concentration at the inlet and the duration of
According to this study, the concentration of carbonate ions increased, there was a corresponding decrease in bicarbonate ions. Conversely, a decrease in carbonate ions resulted in an increase in bicarbonate ions. C.C. Batac et al. demonstrated that the carbonate concentration was constant at zero [11]. At the same time, the concentration of bicarbonate is observed at its Batac et al. demonstrated that the carbonate concentration was constant at zero [11]. At the same time, the concentration of bicarbonate is observed at its highest value in five minutes and then decrease; this sequence is consistent with the reversible reaction between bicarbonate and carbonic acid. highest value in five minutes and then decrease; this sequence is contact. Carbon dioxide can penetrate microalgal cells through active transport mechanism and is subsequently bio fixed. Batac et al. demonstrated that the carbonate concentration was constant at zero [11]. At the same time, the concentration of bicarbonate is observed at its highest value in five minutes and then decrease; this sequence is consistent with the reversible reaction between bicarbonate and carbonic acid. consistent with the reversible reaction between bicarbonate and carbonic acid. Initially, CO2 reacts with water to generate carbonic acid, which then undergoes dissociation into bicarbonate ions (HCO3-) and hydrogen ion (H+). Subsequently, the bicarbonate ions can further dissociate into either carbon dioxide (CO2) or carbonate (CO3-2), contingent upon the pH level of the medium. The concentration of dissolve carbonate and bicarbonate depends on both the CO2 concentration at the inlet and the duration of contact. Carbon dioxide can penetrate microalgal cells through active transport mechanism and is subsequently bio fixed.
According to this study, the concentration of carbonate ions increased, there was a corresponding decrease in bicarbonate ions. Conversely, a decrease in carbonate ions resulted in an increase in bicarbonate ions. C.C. Batac et al. demonstrated that the carbonate concentration was constant at zero [11]. At the same time, the concentration of bicarbonate is observed at its highest value in five minutes and then decrease; this sequence is consistent with the reversible reaction between bicarbonate and carbonic acid.
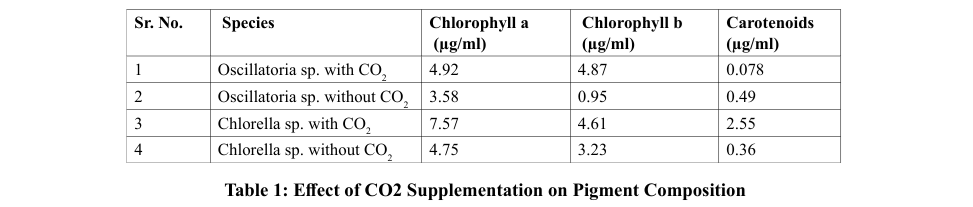
Chlorophyll Pigment
The effects of carbon dioxide on the rate of photosynthesis were investigated by measuring the changes in the photosynthetic pigments. Nitrogen-rich chlorophyll is a substance that promotes cell growth. The process of measuring chlorophyll content was conducted using biomass harvested during a similar phase of cell growth. M.S. Mahajan et al. demonstrated that the strain NCIM 2738 contains 2.601µg/ml Chlorophyll a, 1.308µg/ ml Chlorophyll b and 0.549µg/ml Carotenoids [8]. The strain NCIM 5143 contains 6.924µg/ml Chlorophyll a, 1.336µg/ml Chlorophyll b and 1.768µg/ml Carotenoids. The concentration of chlorophyll a, chlorophyll b and Carotenoids of Chlorella sp. and Oscillatoria sp. was described in Table.
Lipid Content
In the cost-effective production of biofuel from microalgae, both biomass and lipid levels are crucial factors. Carbon dioxide, a key component for photosynthesis, has been found to boost biomass and lipid production when its concentration in the growth medium is increased and its availability improved. Lipid extraction was carried out using the Bligh and Dyer method with some modification. The algal cells were disrupted prior to lipid extraction by Ultra sonification for maximum lipid extraction. In the present study, under CO2 supplementation Chlorella sp. and Oscillatoria sp. had 16.25% and 8.23% of lipid content and without CO2 supplementation, Chlorella sp. and Oscillatoria sp. had 10.29% and 6.87% lipid content, whereas under CO2 supplementation C. pyrenoids NCIM 2738 and S. platensis NCIM 5143 had 21.80 and 45.20 % of lipid content, respectively [8].
Conclusion
The study concluded that the microalgae Oscillatoria sp. and Chlorella sp. are robust microorganism that can tolerate high concentration of CO2 with a range of pH 6 to 10 at temperature 37 °C while giving rise to higher biomass productivity thorough photosynthesis. The biomass productivity was calculated as 80 mg/L for Chlorella sp. and 200 mg/L for Oscillatoria sp. The CO2 sequestration process has the capacity to promote both accelerated and consistent growth in microalgae, facilitating large-scale production, contingent upon advancements in harvesting technology. Lipids, proteins, and polysaccharides found in microalgae biomass with high application value can be utilized as raw materials for the biofuel, biochemical, food, pharmaceutical, and other sectors [12-16].
Acknowledgements
We are thankful to the Department of Biotechnology, Silver Oak Institute of Science, Silver Oak University, Ahmedabad, Gujarat and Gujarat Energy Research and Management Institute, Gandhinagar, Gujarat for providing research work facilities and resources.
References
1. Tomaselli, L. (2004). The microalgal cell. Handbook of microalgal culture: Biotechnology and applied phycology, 1, 3-19.
2. Li, G., Xiao, W., Yang, T., & Lyu, T. (2023). Optimization and process effect for microalgae carbon dioxide fixation technology applications based on carbon capture: a comprehensive review. C, 9(1), 35.
3. Ighalo, J. O., Dulta, K., Kurniawan, S. B., Omoarukhe, F. O., Ewuzie, U., Eshiemogie, S. O., ... & Abdullah, S. R. S. (2022). Progress in microalgae application for CO2 sequestration. Cleaner Chemical Engineering, 3, 100044.
4. Tamburic, B., Evenhuis, C. R., Crosswell, J. R., & Ralph, P. J. (2018). An empirical process model to predict microalgal carbon fixation rates in photobioreactors. Algal research, 31, 334-346.
5. Chakrabarti, T., Krishnamurthi, K., Devi, S. S., & Fulke, B. A. (2018). CO2 sequestration by microalgae: advances and perspectives. Published by OMICS Group eBooks, Foster City.
6. Abraham, J., Prigiobbe, V., Abimbola, T., & Christodoulatos, C. (2023). Integrating biological and chemical CO2 sequestration using green microalgae for bioproducts generation. Frontiers in Climate, 4, 949411.
7. Sudhakar, K., Suresh, S., & Premalatha, M. (2011). An overview of CO2 mitigation using algae cultivation technology. Int J Chem Res, 3(3), 110-117.
8. Mahajan, M. S., Rasheed, M. A., Rao, P. L. S., Bhutiya, P., Hasan, S. Z., & Shah, S. (2021). CO 2 Bio-sequestration Studies on Microalgae—An Approach Through Sustainable Biofuel Production. Macromolecular Characterization of Hydrocarbons for Sustainable Future: Applications to Hydrocarbon Value Chain, 275-286.
9. Mokashi, K., Shetty, V., George, S. A., & Sibi, G. (2016). Sodium bicarbonate as inorganic carbon source for higher biomass and lipid production integrated carbon capture in Chlorella vulgaris. Achievements in the Life Sciences, 10(1), 111-117.
10. Zhu, C., Zhai, X., Xi, Y., Wang, J., Kong, F., Zhao, Y., & Chi, Z. (2020). Efficient CO2 capture from the air for high microalgal biomass production by a bicarbonate Pool. Journal of CO2 Utilization, 37, 320-327.
11. Batac, C. C., Gathercole, N. S., Maravilla, A. F., & Beltran, A. B. (2020, April). Evaluation of different carbonate sources for bicarbonate-based integrated carbon capture and algae production system using Spirulina platensis. In IOP Conference Series: Materials Science and Engineering (Vol. 778, No. 1, p. 012041). IOP Publishing.
12. Cordoba-Perez, M., & de Lasa, H. (2021). CO2-Derived Carbon Capture Using Microalgae and Sodium Bicarbonate in a PhotoBioCREC Unit: Kinetic Modeling. Processes, 9(8), 1296.
13. Eloka-Eboka, A. C., & Inambao, F. L. (2017). Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Applied Energy, 195, 1100 1111.
14. Iglina, T., Iglin, P., & Pashchenko, D. (2022). Industrial CO2 capture by algae: a review and recent advances. Sustainability, 14(7), 3801.
15. Oo, Y. N., Su, M. C., & Kyaw, K. T. (2017). Extraction and determination of chlorophyll content from microalgae. International Journal of Advanced Research and Publications, 1(5), 298.
16. Prasad, R., Gupta, S. K., Shabnam, N., Oliveira, C. Y. B., Nema, A. K., Ansari, F. A., & Bux, F. (2021). Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability, 13(23), 13061.



