Research Article - (2025) Volume 3, Issue 1
Mitochondria Cannot be the Powerhouse of the Cell. The Unsuspected Intrinsic Capacity of Eukaryotic Cell to Dissociate the Water Molecule, Like in Plants
Received Date: Feb 14, 2025 / Accepted Date: Mar 17, 2025 / Published Date: Mar 28, 2025
Copyright: ©©2025 Arturo Solís Herrera, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Herrera, A. S., Esparza, M. D. C. A. (2025). Mitochondria Cannot be the Powerhouse of the Cell. The Unsuspected Intrinsic Capacity of Eukaryotic Cell to Dissociate the Water Molecule, Like in Plants. J Water Res, 3(1), 01-08.
Abstract
Sunlight is the most abundant energy source on this planet. However, the ability to convert sunlight into biological energy in the supposed form of adenosine-5 Ì-triphosphate (ATP) could be a wrong belief, because it is limited to chlorophyll-containing chloroplasts in photosynthetic organisms.
The mammalian mitochondria can also reaction to light and increase significantly synthesis of ATP when mixed with a light- capturing metabolite of chlorophyll. the same potential to convert light into energy exists in mammals, as chlorophyll metabolites accumulate in mice, rats and swine when fed a chlorophyll-rich diet. So, through consumption of plant chlorophyll pigments, animals, too, can derive energy directly from sunlight.
Our finding about the presence of several molecules in the Eukaryotic cell like melanin and those molecules derived from Protoporphyrin IX (PTP IX) that can dissociate the water molecules break the ground about our artificial division on Photosynthetic and Non-photosynthetic organisms. It turns out that all living beings can dissociate water molecules at the intracellular level, given that the cellular needs for oxygen (and hydrogen) are very demanding and constant, and with this capacity, hitherto unsuspected, each cell in our body produces its own oxygen (and hydrogen).
Keywords
ATP, Chlorophyll, Energy, Hydrogen, Melanin, Mitochondria, Photosynthesis, Protoporphyrin IX, Oxygen, Water dissociation
Background
Determining how organisms obtain energy from the environment is fundamental to our understanding of life. Currently, it is believed that in nearly all organisms, energy is stored, although Energy cannot be stored; and then transported as adenosine-5´- triphosphate (ATP) [1]. In animals, the vast majority of ATP is synthesized in the mitochondria through respiration, a catabolic process. And on the other hand, it is a deep-rooted dogma, originating since the mid-seventeenth century, that plants have co-evolved endo-symbiotically to produce chloroplasts, which synthesize light-absorbing chlorophyll molecules that can capture light to use -supposedly- as energy for ATP synthesis.
Currently, it is considered that the energetic demand during normal physiology involves both glycolysis and mitochondrial oxidative phosphorylation. But both metabolic processes have been interpreted so far, based on the theoretical fact that glucose is a source of energy, and that our body obtains oxygen from the air we breathe.
However, both processes are based on beliefs dating back to the mid-eighteenth century, and which to date have not been proven [2].
Carbon Dioxide
CO2 plays an important role in mitochondrial metabolism. The enzymes of the tricarboxylic acid cycle that produce CO2 are located within the mitochondrial matrix, as are those enzymes that fix CO2 in the pathways of gluconeogenesis and urea production [3-5].
Prior to 1972, the few studies of the interaction of CO2 and HCO3- with mitochondria dealt with the permeability of the inner mitochondrial membrane to these two species and with the possible existence of a mitochondrial carbonic anhydrase. Chappell and Crofts showed that the inner mitochondrial membrane was essentially impermeable to HC03- but readily permeable to C02, a finding now widely accepted [6,7].
Experimental results are parallel to those seen with lysed, as compared with intact, erythrocytes; they strongly imply that liver mitochondria have carbonic anhydrase activity in the matrix [8]. Liver mitochondria have significant carbonic anhydrase; skeletal muscle mitochondria have about one-third as much. Heart, brain, and kidney mitochondria have no detectable carbonic anhydrase activity.
Rat renal tubular cells contain a high content of carbonic anhydrase, 97% of it cytosolic, with the remainder in the brush border and basal-lateral regions of the plasma membrane [9]. Sonication of isolated liver mitochondria yielded a soluble fraction containing the enzyme, confirming the conclusion drawn from C160180 exchange that this enzyme is in the mitochondrial matrix [10].
The C160180 exchange technique provides a convenient method for estimating the pH of the mitochondrial matrix space. It was independent of mitochondrial protein concentration in the reaction medium. In intact mitochondria, the activity was always higher than that observed in broken mitochondria at a given pH of the medium, implying that the mitochondrial matrix was more alkaline [11].
The exchange of 180 from C160180 into H 160, as measured by mass spectrometry, is the only technique of which we are aware that can directly determine carbonic anhydrase activity inside a cell or vesicle. Among the many doubts about carbonic anhydrase function in different organs and physical / chemical properties, is in regards of its energy source, and since, as the suffix “ase” in its name indicates, it does not use ATP to carry out its function, then the molecular hydrogen that is generated by dissociating the molecule of water would be an ideal candidate.
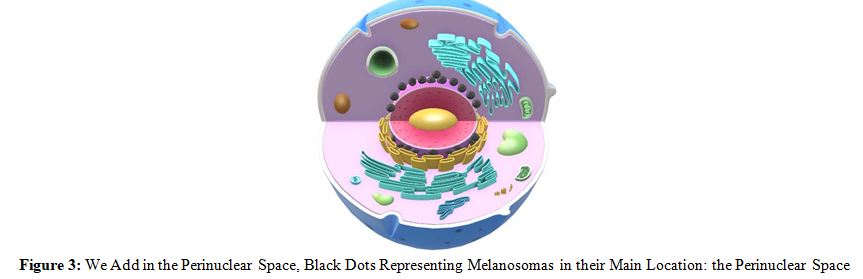
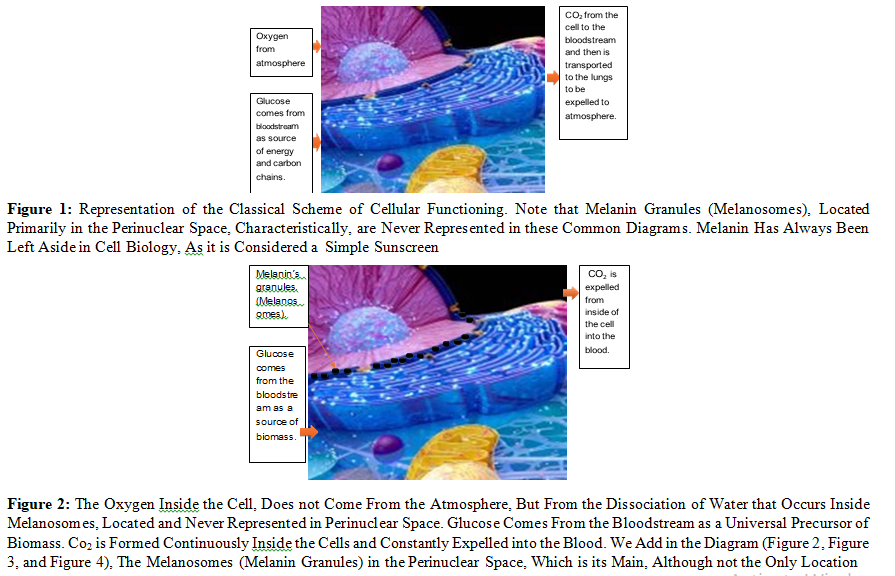
So, to the traditional schemes (Figure 1), which pretend to represent the processes of cell biology, we would have to eliminate the arrow that indicates that oxygen comes from the blood, and from now on, emphasize that oxygen (and hydrogen) come from the dissociation of water that takes place inside the cell (Figure 2, Figure 3, and Figure 4). mainly by melanin, although other molecules also carry out this process, for example: all derivatives of protoporphyrin IX (PTP IX).
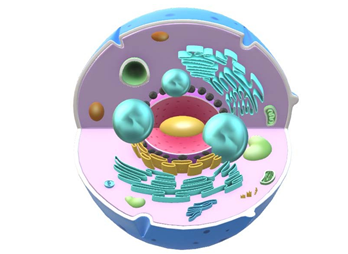
Figure 4: The Blue Spheres Represent the Molecular Oxygen and Hydrogen Released by Melanosomes when the Molecule Dissociates from the Water Inside, and Which Move Throughout the Interior of the Cell, as Growing Spheres. During their Displacement, the Organelles Capture Hydrogen with its Precious Energy Charge, the First Organelles to Benefit are the Cell Nucleus, which does not have Mitochondria or ATP, and then the Rough Endoplasmic Reticulum, Which to Capture the Greatest Amount of Hydrogen and Oxygen, Surrounds the Cell Nucleus, Forming an Almost Hermetic Envelope Around the Nucleus
The unsuspected ability of eukaryotic cells to dissociate water molecules, through various molecules such as melanin and those derived from la protoporphyrin IX (PTP IX) represent a revolution of biblical proportions, since it involves rewriting textbooks, almost from scratch (Figure 5).
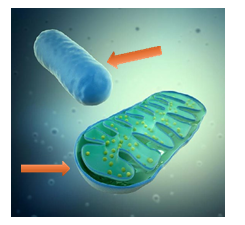
Figure 5: The Oxygen (and Hydrogen) that Penetrates the Mitochondria (Upper Arrow) do not come from the Atmosphere, but from the Dissociation of Water that Takes Place Inside Each Cell. The Bottom Arrow Represents Glucose or its Intermediate Metabolites, and they Come From the Bloodstream
Under current dogmas, Oxygen inside the cell comes from the atmosphere absorbed through the lungs, then blood stream, blood vessels, etc., and CO2 is formed inside the cell from where it is expelled into the interstitial space, blood vessels, and reaches the lungs through the bloodstream, and then expelled through the alveolar walls into the alveolar space, and then expelled into the atmosphere using the inspired air as a vehicle.
But the finding that eukaryotic cells (and prokaryotes) possess molecules capable of transforming the power of light into chemical energy that can be used by cells, that is, through the dissociation of water molecules, means that each cell in our body can oxygenate itself. So, in the "new" dogma, we would have that both oxygen and carbon dioxide come from inside the cell.
The unsuspected ability of the eukaryotic cell to transform the power of light into chemical energy through the dissociation of water, like plants, tells us that the cells are energetically independent of each other, but to the replenishment of wearied or wasted molecules, they do depend on the intake of food, mainly glucose, considered the universal precursor of any organic molecule, both in animals and plants. Thereby glucose is a source of biomass but not of energy.
Therefore, we can no longer consider the mitochondria as the powerhouse of the cell, since its energy supply fully depends, like any other intracellular organelle, on the dissociation of water that occurs in all the cells of the body. The functions of the mitochondria are more related to the accurate control of temperature and phosphate levels.
Current Physiology of Mitochondria
Currently, the concept is that Mitochondria generate energy as electrons are passed from donors at lower to acceptors at higher redox potential through various protein complexes. Along with this process, protons are pumped from the matrix outward, generating a potential difference across the inner membrane. The resulting potential energy is transferred to ATP or dissipated as heat as protons leak back toward the matrix.
Despite an enormous amount of literature and the importance of the problem, very little information is available about the structural features of mitochondrial cation channels and exchangers, whereas a vast amount of information is available about the functional properties of these mitochondrial transport systems.
According to Mitchell's 4 postulates in which he thought his chemiosmotic theory could be proven, are mentioned the membrane-located ATPase systems of mitochondria [12]. But the suffix ase (ATPase) means that this enzyme does not use ATP as an energy source, and Mitchell does not specify where this energy would come from, which is undoubtedly essential for ATPase systems located in the mitochondrial membrane to function properly. So, the dissociation of water, which occurs mainly in the perinuclear space, may be the explanation, since when the molecule dissociates from water, oxygen and hydrogen are generated simultaneously.
And the latter is the carrier of energy, par excellence, in the entire universe and not only inside cells. So, part of the energy that is released during the dissociation of the molecule from water is trapped by it, and then transported, mainly following the laws of simple diffusion.
Thereby mitochondria neither can nor needs generate energy, since the dissociation of water molecules, which mainly takes place in the perinuclear space, releases enough energy when the water molecule breaks, and a significant part of this energy is captured by molecular hydrogen that is released along with oxygen, also in molecular form (Figure 6).
Hydrogen is the main carrier of energy in the entire universe, and therefore inside the cells, therefore hydrogen energetic behavior cannot be different, thereafter, molecular hydrogen (H2(gas)) carries energy following the laws of simple diffusion, extending to ways of increasing spheres that are directed towards the cell membrane and at the same time to the nucleus, constituting their main or perhaps only source of energy since the cell nucleus does not have mitochondria or ATP molecules.
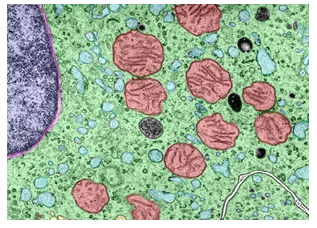
Figure 6: Under the New Dogma, Oxygen (and Hydrogen) is Generated Mainly in the Perinuclear Space (Left), Since it is the Usual Location of Melanosomes. And from there they go to both the Cytoplasm, Meeting Both the Oxygen and Hydrogen Needs of all Organelles, Including Mitochondria (Right) Reaching the Cell Membrane, And Since Melanosomes Envelop the Nucleus of the Cell, They Also go to it. Hydrogen is the Smallest Atom (7 Nm), So it Easily Crosses the Membranes of the Mitochondria and Any Organelle. It is Also the Fastest Moving Atom or Even Molecule. Source of Photograph: Histology Guide. Virtual microscopy laboratory. Histologyguide.com
Changes in the pH of the cell, and of any chemical reaction, presuppose energy exchange, and the more marked they are, the greater the intensity of energy exchange.
Mammal Erythrocytes do not Have Mitochondria
Red blood cells (RBCs) or erythrocytes are continually formed in the bone marrow. RBCs originate from nucleated stem cells, which mature into nucleated erythroblasts, then differentiate into anucleate reticulocytes, and finally into red blood cells (RBC). RBCs are terminally differentiated cells (they cannot divide anymore) and are shed from the bone marrow into the blood circulation. In contrast to mammals, RBCs in birds, reptiles, and other lower vertebrates have nuclei.
The anucleate erythrocyte, as it is seen in mammals, is considered more evolutionarily advanced [13]. In addition to the differences in the circulatory system, mammals have smaller end-blood-vessels (capillaries of about 3 μm in diameter) than birds.
To squeeze through these small blood capillaries and make maximum surface for hemoglobin, RBC (about 8 μm in diameter for human and 10–15 μm in diameter for birds) must be very flexible [14]. The presence of a nucleus may prevent big, nucleated RBC from squeezing through these small capillaries.Therefore, during the evolutionary development, nature has found that it was better to extrude the nucleus and other cell organelles, such as endoplasmic reticulum for protein synthesis, which were not needed for their actual function as oxygen carrier, (if any).
However, nucleus in the erythroblast is about 2 μm in diameter [15]. And, if it was distributed at peripherally inflated region of the erythrocyte, it would neither hinder erythrocyte eformation nor its entrance into end-blood-vessels. (Figure 7).
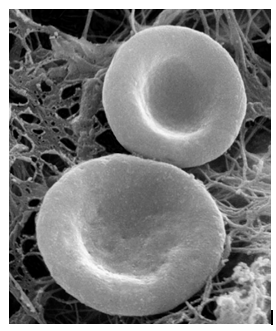
Figure 7: The Biconcave Shape of Erythrocytes is Due to the Loss of the Cell Nucleus, As Well as the Absence Of Mitochondria, Endoplasmic Reticulum, and Others. And the vacuum or Negative Pressure Generated by Hemoglobin When it Irreversibly Dissociates the Water Molecules Contained Inside the Erythrocyte, and this Negative Pressure or Vacuum is Intense Enough for the Erythrocyte Cell Membrane to "Copy" the Interior Morphology of the Erythrocyte. Source of Photograph: Histology Guide. Virtual Microscopy Laboratory. Histologyguide.com
In relation to the passage of erythrocytes through capillaries of 3 um, it cannot be possible due to the force of contraction of the left ventricle, because if it were, we would need a heart about 25 times larger (Gerard Pollack, unpublished data). Nor does the erythrocyte carry oxygen from the lung to the tissues, in principle because atmospheric oxygen does not pass through the lung tissue due to its high-water content, so it repels it. Chlorophyll and hemoglobin are more than 90% similar, in addition to the fact that both molecules come from the same precursor: protoporphyrin IX (PTP IX), a molecule present in all living beings. Therefore, both molecules irreversibly dissociate the water molecules.
Mitochondria are wrongly known as the powerhouses (batteries) of the cell, although they are not related to oxygen carriers. So, there is no sound reason to abandon mitochondria for the living cells either. We think that the function of mitochondria, rather than the production of energy from glucose and derivatives, is the regulation of temperature inside cells, as well as the fine control of phosphate levels also inside the cell, given its relatively high toxicity. Fair-skinned people who live in cold climates have up to 83% more mitochondria than dark-skinned people who live in warm climates.
Severe hemolysis or miolysis occurring during pathological states, such as sickle cell disease, ischemia reperfusion (IR), and malaria, results in high levels of free heme, also causing severe ROS accumulation [16]. So, it seems that lacking nucleus and mitochondria benefits mammal erythrocytes against sugar/heme- induced oxidative stress.
However, just as chlorophyll is inactivated in less than 20 seconds when extracted from plant cells, hemoglobin (and myoglobin) is also able to dissociate water molecules only when they are intracellular, inactivating rapidly when for one reason or another they are not there or lose their natural location. Water dissociation at room temperature is an exact process, amazingly exact, so in addition to pH, temperature, electrolytes, etc., intracellular localization is within strict ranges.
Therefore, the toxic effects of the heme group are more related to the loss of function than to the toxicity of the molecule itself, since the blood is a chemical laboratory in motion that requires oxygen and hydrogen, to carry out the also precise chemical reactions and changes suffered by the biological molecules that the cells of the body pour into the bloodstream. where they finish being chemically conditioned so that they are biologically active.
Finally, if the mitochondria were a source of energy, mammal erythrocytes would not be able to carry out their function or even preserve their shape, since even to preserve shape requires energy.
Oxidative Phosphorylation
Also known as electron transport-linked phosphorylation, refers to a theoretical metabolic pathway in which the energy released by nutrients during oxidation is utilized to generate ATP through electrical transport chain. Oxidative phosphorylation provides bulk ATP for living organisms, and the ATP (theoretically) is the main energy source for maintaining life activity. But mitochondria, ATP, ADP, and AMP, are more of a way by which the temperature inside the cell remains constant and at the same time controls phosphate levels, given their high toxicity.
The term electron transport "chain" has been criticized as misleading, because it implies a linear progression along a single pathway, which is probably the most real possibility, given that electrons travel at speeds close to the speed of light, and controlling the direction of the electron at such speeds is by no means straightforward. Furthermore, the electrons are easily and quickly absorbed.
So, it is more congruent that the source of energy for cells is the hydrogen that is released along with oxygen when the water molecule breaks down. And because it is the smallest atom (7.0 nm), hydrogen easily permeates through the various membranes that the cell contains, constituting the source of energy even for the mitochondria itself, including the mitochondrial genome contained deep inside it.
Experimental evidence also exists suggesting that the cell nucleus may exert a negative control on the mitochondrial genome through some short-lived nuclear substance(s) [17]. Interestingly, the cell nucleus does not contain mitochondria. A potential link between nuclear and mitochondrial DNA replication also has been described in Drosophila, suggesting a common regulatory mechanism controlling nuclear and mitochondrial DNA replication, for instance the energy transported by the hydrogen coming from water dissociation that happens inside melanosomes [18].
The multiple mtDNA deletions in patients with progressive ex- ternal ophthalmoplegia suggest widespread damage, character- istic of power failures [19]. The link between mitochondrial and nuclear DNA replication is probably very complex and regulated by additional factors such as mRNA levels for mtSSB (MITO- CHONDRIAL SINGLE-STRAND BINDING PROTEIN) varies independently of cell proliferating activity [20]. Because of its probable prokaryote origin, in many aspects, the mitochondrion behaves as an independent entity living inside a eukaryotic cell. All basic processes associated with life (DNA maintenance, tran- scription, and translation) occur inside the organelle. However, most of the factors involved in promoting and controlling these processes are borrowed from the cytoplasm, where nuclear-coded proteins are synthesized.
Our understanding of the intricate relationships between mitochondrial and nuclear genomes is still limited, due mainly to the fact that genetic manipulation and in vitro systems are difficult to develop for organelles. Nevertheless, a picture is emerging that shows the mitochondrial genetic system has many features in common with the putative prokaryote ancestral yet has developed a few unique mechanisms as it evolved as an endosymbiont taking advantage of what a complex eukaryote nuclear genome has to offer [21].
The mitochondrial properties differ among populations of Atlantic killifish (Fundulus heteroclitus) in the liver, but not in brain or heart, clearly indicating that mitochondrial properties are not necessarily equivalent among tissues [22]. Testing how sensitive in vitro mitochondrial function is to change in physiologically or ecologically relevant abiotic factors, particularly variables such as temperature, ion concentrations, and both substrate and oxygen availability. These variables are expected to vary within an animal’s environment and may fluctuate in the cytosol in cells of ectotherms and endotherms, Osmo conformers, as well as animals at altitude or depth [23].
There is increasing evidence that mitochondria play a critical role in the survival and performance of animals via their capacity to adapt their function to meet the challenges imposed by environmental variation [24]. The observed increases in mitochondrial abundance arose primarily from an enrichment of subsarcolemmal mitochondria, the subpopulation located closest to capillaries, which may be advantageous for mitochondrial O2 supply, however, oxygen does not come from the blood vessels, but from the inside of each cell, and it is the cell itself that consumes it, expelling the excess oxygen to the outside of the cell, but in the form of CO2, which is about 25 times more soluble in water than oxygen, for which it simply adds a carbon atom, probably coming from the metabolism of food.
The adaptive variation in important mitochondrial phenotypes can be tissue specific. The amazing plasticity in mitochondrial function that has evolved within Animalia emphasizes the important role of mitochondrial plasticity in animals’ tolerance of environmental change. But such plasticity requires energy to happen properly, and the mitochondria cannot supply it to itself, it is more congruent that the oxygen and hydrogen requirements are based on the dissociation of water.
It confirmed the importance of aerobic scope in the evolution of endothermy: absolute aerobic scope, ATP generation by mitochondria and muscle power output are all strongly temperature- dependent, indicating that there would have been significant improvement in whole-organism locomotor ability with a warmer body [25]. Large dinosaurs were warm, but were not endotherms, and the metabolic status of pterosaurs remains unresolved.
The erythrocyte size is empirically tightly linked to endothermy [26]. Small erythrocytes were identified as a key feature for extant endotherms because their small size and globular shape increases their exchange surface and the reduction (birds) or total removal (mammals) of their nuclear content allows them to deform more easily. These erythrocyte features permit a higher gas exchange efficiency compared to the larger erythrocytes of ectothermic vertebrates. Such a cell size reduction is associated with a diminution of the capillary diameters, as the erythrocytes must be larger than the capillaries, they are passing through to deform and increase the oxygen delivery rate [27].
Almost all the species are found ectothermic and ancestral state reconstructions strongly support an ancestral ectotherm in amniotes. A four chambered heart is commonly associated with endothermy. Endothermy can hardly be defined as a unique feature, but rather as the acquisition of various features, a kind of “endothermic system” .
The higher concentration of oxygen (and hydrogen) in the tissues of the eye (and body) correlates with the greater presence of melanin, not blood vessels. Bloodstream inside blood vessels carry CO2 but not oxygen. Water repels gases, hence the low partial pressure of oxygen in the vitreous body, was to be expected.
The observation that each cell dissociates the water molecule and is therefore capable of producing its own oxygen (and hydrogen) presupposes a new beginning for the subject of biology and medicine (Figure 8).
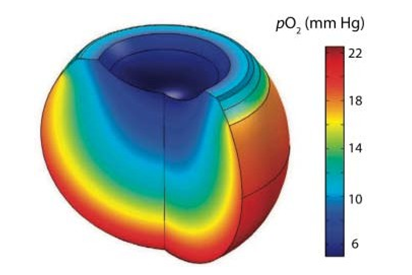
Figure 8: Mathematical Model of Oxygen Distribution in the Vitreous Body. From Filas Et Al: “Computational Model for Oxygen Transport and Consumption in Human Vitreous”. @ ARVO. The Part with the Highest Po2 (Mm Hg), In Red, Coincides with The Anatomical Location of the Uveal Tract of the Eyeball, Which Means Grape-Colored, Due to its High Melanin Content, Usually 40% More Than in the Skin.
Conclusion
The role of mitochondria requires rethinking the finding that oxygen comes from inside the cell itself and not from the outside. Each cell has molecules capable of transforming the power of light into chemical energy, by dissociating water, like plants. Banishing an eighteenth-century concept will mark before and after in the biology of the mitochondria, the cell, the human body, and biology.
Conflict of Interest:
None.
References
1. Xu, C., Zhang, J., Mihai, D. M., & Washington, I. (2014). Light-harvesting chlorophyll pigments enable mammalian mitochondria to capture photonic energy and produce ATP. Journal of cell science, 127(2), 388-399.
2. Stobbe, M. D. (2012). The road to knowledge: from biology to databases and back again (Doctoral dissertation).
3. Greenberg, D. M. (1960). Metabolic pathways. Volume 1.
4. Racker, E. (Ed.). (1894). Membranes of mitochondria and chloroplasts (No. 165). Van Nostrand Reinhold Company.
5. Azzone, G. F. (Ed.). (1972). Biochemistry and Biophysics of Mitochondrial Membranes: Proceedings. Academic Press.
6. Papa, S., Quagliariello, E., & Slater, E. C. (1966). Regulation of metabolic processes in mitochondria (p. 180). J. M. Tager (Ed.). Amsterdam: Elsevier.
7. Sarkadi, B., Tosteson, D. C., Giebisch, G., & Ussing, H. H. (1979). Membrane Transport in Biology. G. Giebisch, DC Tosteson.
8. Itada, N., & Forster, R. E. (1977). Carbonic anhydrase activity in intact red blood cells measured with 18O exchange. Journal of Biological Chemistry, 252(11), 3881-3890.
9. Wistrand, P. J., & Kinne, R. (1977). Carbonic anhydrase activity of isolated brush border and basal-lateral membranes of renal tubular cells. Pflügers Archiv, 370, 121-126.
10. Wood, H. G., & Utter, M. F. (1965). The role of CO2 fixation in metabolism. Essays in biochemistry, 1, 1-27.
11. Tischler, M. E., Hecht, P., & Williamson, J. R. (1977). Deter- mination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Archives of biochem- istry and biophysics, 181(1), 278-292.
12. Mitchell, P. (1966). Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biological Reviews, 41(3), 445-501.
13. Snyder, G. K., & Sheafor, B. A. (1999). Red blood cells: centerpiece in the evolution of the vertebrate circulatory system. American zoologist, 39(2), 189-198.
14. Park, Y., Best, C. A., Auth, T., Gov, N. S., Safran, S. A., Popescu, G., ... & Feld, M. S. (2010). Metabolic remodeling of the human red blood cell membrane. Proceedings of the National Academy of Sciences, 107(4), 1289-1294.
15. Ji, P., Jayapal, S. R., & Lodish, H. F. (2008). Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nature cell biology, 10(3), 314-321.
16. Kumar, S., & Bandyopadhyay, U. (2005). Free heme toxicity and its detoxification systems in human. Toxicology letters, 157(3), 175-188.
17. Rinaldi, A. M., De Leo, G., Arzone, A., Salcher, I., Storace, A., & Mutolo, V. (1979). Biochemical and electron microscopic evidence that cell nucleus negatively controls mitochondrial genomic activity in early sea urchin development. Proceedings of the National Academy of Sciences, 76(4), 1916-1920.
18. Lefai, E., Calleja, M., Ruiz de Mena, I., Lagina III, A. T., Kaguni, L. S., & Garesse, R. (2000). Overexpression of the catalytic subunit of DNA polymerase γ results in depletion of mitochondrial DNA in Drosophila melanogaster. Molecular and General Genetics MGG, 264, 37-46.
19. Van Goethem, G., Dermaut, B., Löfgren, A., Martin, J. J., & Van Broeckhoven, C. (2001). Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nature genetics, 28(3), 211-212.
20. de Mena, I. R., Lefai, E., Garesse, R., & Kaguni, L. S. (2000). Regulation of Mitochondrial Single-stranded DNA-binding Protein Gene Expression Links Nuclear and Mitochondrial DNA Replication inDrosophila. Journal of Biological Chemistry, 275(18), 13628-13636.
21. Moraes, C. T., Srivastava, S., Kirkinezos, I., Oca-Cossio, J., Woischnick, M., & Diaz, F. (2002). Mitochondrial DNA structure and function. International review of neurobiology, 53, 3-23.
22. Chung, D. J., Healy, T. M., McKenzie, J. L., Chicco, A. J., Sparagna, G. C., & Schulte, P. M. (2018). Mitochondria, temperature, and the pace of life. Integrative and Comparative Biology, 58(3), 578-590.
23. Hood, W. R., Austad, S. N., Bize, P., Jimenez, A. G., Montooth, K. L., Schulte, P. M., ... & Salin, K. (2018). The mitochondrial contribution to animal performance, adaptation, and life- history variation. Integrative and Comparative Biology, 58(3), 480-485.
24. Scott, G. R., Guo, K. H., & Dawson, N. J. (2018). The mitochondrial basis for adaptive variation in aerobic performance in high-altitude deer mice. Integrative and comparative biology, 58(3), 506-518.
25. Clarke, A., & Pörtner, H. O. (2010). Temperature, metabolic power and the evolution of endothermy. Biological Reviews, 85(4), 703-727.
26. Soslau, G. (2020). The role of the red blood cell and platelet in the evolution of mammalian and avian endothermy. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 334(2), 113-127.
27. Snyder, G. K., & Sheafor, B. A. (1999). Red blood cells: centerpiece in the evolution of the vertebrate circulatory system. American zoologist, 39(2), 189-198.




