Research Article - (2025) Volume 10, Issue 1
Fragmented QRS Predicts 2-Year Mortality in Patients Undergoing Transfemoral-Transcatheter Aortic Valve Replacement: A Retrospective Observational Study
Received Date: Apr 01, 2025 / Accepted Date: Apr 19, 2025 / Published Date: Apr 25, 2025
Copyright: ©©2025 Shogo Tsujikawa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Yoshimura, M., Tsujikawa, S., Takuno, Y., Hino, H., Hori, K., et al. (2025). Fragmented QRS Predicts 2-Year Mortality in Patients Undergoing Transfemoral - Transcatheter Aortic Valve Replacement: A Retrospective Observational Study. Cardio Open, 10(1), 01-08.
Abstract
Background: Transcatheter aortic valve replacement (TAVR) is a well-established therapeutic strategy in high-risk patients with severe aortic stenosis. The aim of this study was to analyze the prognostic value of the fragmented QRS morphology (fQRS) on 12-lead electrocardiography (ECG) for predicting 2-year mortality in patients who underwent TAVR.
Methods: A total of 272 patients (median age; 84 years, 68.4% female) undergoing the TAVR approached from the femoral artery between 2016 and 2020 were included in this retrospective cohort study. Patients were assigned into two groups based on the presence or absence of fQRS on 12-lead ECG before the procedures. Independent predictors of 2-year mortality were evaluated using Cox regression and Kaplan-Meier survival analysis.
Results: During the study period, in total, 272 patients were assigned into two groups based on the presence (n = 108) and the absence (n = 164) of fQRS on 12-lead ECG before TAVR procedures. All-cause mortality was higher in the fQRS group (34 [31.5%]) than in the non-fQRS group (5 [3%]) during a mean follow-up of 21.9 months. Kaplan-Meier survival analysis revealed significantly lower all-cause mortality in the non-fQRS group (p < 0.001). The independent predictor of 2-year mortality at follow- up in multivariate Cox regression analysis was the presence of fQRS (hazard ratio, 1.371; 95% confidence interval, 1.072–1.754; p = 0.012). Severe mitral valve regurgitation and paravalvular leakage did not independently predict 2-year mortality.
Conclusions: The presence of fQRS on 12-lead ECG was an independent predictor of 2-year survival in patients undergoing TAVR. The fQRS may serve as a valuable factor to identify patients with higher risk who will need close follow-up and more intense treatment after TAVR surgery.
Keywords
Fragmented QRS, Transfemoral Aortic Valve Replacement (TAVR), Aortic Stenosis (AS)
Abbreviations
TAVR: Transcatheter Aortic Valve Replacement SAVR: Surgical Aortic Valve Replacement fQRS: Fragmented QRS
ECG: Electrocardiography
AS: Aortic Stenosis
STEMI: ST Elevation Myocardial Infarction
EuroSCORE: European System for Cardiac Operative Risk Evaluation
Introduction
Aortic stenosis (AS) has become an increasingly common health problem, especially among the older population [1]. The most common treatment method used in these patients is surgical aortic valve replacement (SAVR). However, compared with SAVR, transcatheter aortic valve replacement (TAVR) is a minimally invasive useful approach for AS in patients with high surgery risk. Although SAVR remains the gold standard for the treatment of severe AS, recent studies have shown less short-term mortality after TAVR in high-risk patients [2]. Thus, the patients who are TAVR candidates possess more risk factors and comorbidities. In advanced AS, chronic pressure overload causes myocardial fibrosis and infarction, progressing from left ventricular hypertrophy to heart failure [3]. Some studies show that myocardial fibrosis and infarction in patients with AS are related to poor outcomes even after aortic valve replacement [4].
Fragmented QRS (fQRS) is a 12-lead surface electrocardiographic finding related to conduction abnormalities because of myocardial scarring [5]. The association between fQRS and all-cause mortality has been shown in various clinical situations such as heart failure and ST elevation myocardial infarction (STEMI) [6,7]. Additionally, the presence of fQRS on electrocardiography (ECG) before surgery may provide predictive information about the long-term mortality in patients undergoing isolated SAVR for severe AS [8,9]. The outcome of TAVR can be influenced by various predictors like SAVR, and accurate risk assessment plays an important role in perioperative management. However, the impacts of the fQRS on ECG and TAVR outcomes have not been extensively studied. The fQRS has emerged as an easily obtainable predictor derived from routine preoperative ECG. Therefore, this study aimed to determine the predictive effect of fQRS morphology on 2-year survival and to identify correlates of 2-year death in patients undergoing transfemoral TAVR.
Method
Study Design and Population
In this observational study, 291 patients with severe AS, who were scheduled for elective transfemoral TAVR between January 2016 and February 2020, were included. Patients with a pre-existing paced rhythm on ECG or who needed complex surgeries were excluded. This study was approved by the Ethics Committee of Osaka Metropolitan University (approval number: 2021- 175) and registered with the Japan Registry of Clinical Trials (jRCT1050240304). The need for informed consent was waived because the data analyses were performed retrospectively. In addition, those patients in whom 12-lead ECG data could not be obtained were excluded from the study. Patients were assigned into two groups based on the presence (fQRS group: n = 108) or the absence (non-fQRS group: n = 164) of fQRS. Baseline demographic and clinical characteristics were recorded for all patients. Routine laboratory tests, standard 12-lead ECG, and transthoracic echocardiography were performed on all patients. In addition, postoperative clinical data, including the time of death, were evaluated in the outpatient department during the study period.
Electrocardiographic Measurements
A standard 12-lead ECG was recorded with each patient at rest in the supine position, using a paper speed of 25 mm/s and an amplitude of 10 mm/mV from all patients before TAVR. All ECG recordings were interpreted by two independent clinicians, blinded to the patients’ clinical information. In case of conflict, the final decision was made by consensus. Fragmented QRS was defined as the presence of various RSR’ patterns (QRS duration < 120 msec) with or without the Q wave, which include an additional R wave (R’) or notching of the R wave or the S wave, or the presence of > 1 R’ (fragmentation) without typical bundle branch block in two contiguous leads corresponding to a major coronary artery territory [10]. Typical right and left bundle branch block patterns (QRS duration > 120 msec) and incomplete right bundle branch block patterns (QRS duration between 100 msec and < 120 msec) in leads V1 or V2 were excluded from the study. An example of fQRS on ECG is shown in Figure 1.
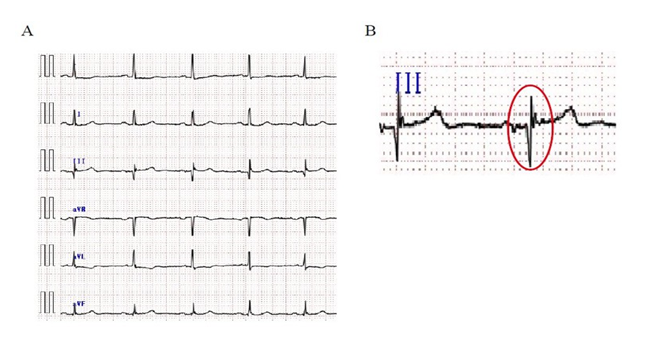
Figure 1: (A) Examples of Fragmented QRS on Leads II, III and aVF in Electrocardiography. (B) Examples of Fragmented QRS which include an Additional R Wave on Leads III at High Time Resolution
Outcomes
The primary outcome of this study was 2-year mortality, which was defined as death resulting from any cause within 2 years after elective TAVR surgeries. The secondary outcomes were the length of stay in hospital, and postoperative complications, such as new postoperative stroke (defined as focal brain injury with a permanent functional deficit), high-degree atrioventricular block (defined as need for permanent pacemaker), need for reintubation (defined as respiratory and/or heart failure), and delirium.
Statistical Analysis
Statistical analysis was calculated using SigmaPlot version 14 (Systat Software) with a confidence level of 95%, and results with p < 0.05 were considered statistically significant. As this was a retrospective observational study, a formal power analysis was not performed. Normally distributed continuous parameters are presented as mean ± standard deviation and skewed continuous parameters are expressed as median and interquartile range. Categorical data are presented as numbers and percentages and are compared using χ2 test or Fisher’s exact test, as appropriate to identify statistically significant differences. Parametric test assumptions were checked before the analysis. After the normality of the variables was tested by Shapiro–Wilk test, Student’s t-test or Mann–Whitney U-test, as appropriate, was used to compare the independent samples. Overall survival estimates were performed using the Kaplan–Meier method. All clinical variables were tested individually by univariate log-rank analysis. Variables with a p-value < 0.05 were included in a multivariate Cox proportional hazard model to assess the associated variables. Overall survival was defined as the time elapsed between the date of TAVR surgeries and death from any cause; patients alive at the last follow up were censored. Hazard ratios and 95% confidence intervals were calculated to identify the risk factors.
Results
Data from 291 consecutive patients who underwent TAVR procedures between January 2016 and February 2020 were available in our registry. Data of 19 patients who had pre-existing paced rhythm on ECG before TAVR or need for complex surgeries were excluded from analysis. Ultimately, 272 patients who underwent TAVR were enrolled in this study (Figure 2). Patients were followed for up to 2 years (range: 1 day to 2 years). These patients were divided into 2 groups according to the presence or absence of fQRS on preoperative ECG. Baseline characteristics and risk factors of patients are summarized in Table 1, showing no substantial difference in basic characteristics between the two groups including the data of transthoracic echocardiography before TAVR procedures.
|
|
fQRS (n = 108) |
non-fQRS (n = 164) |
p value |
|
Age (years) |
83.4 ± 5.8 |
83.9 ± 5.0 |
0.751 |
|
Female, n (%) |
71 (65.7) |
115 (70.1) |
0.531 |
|
Height (cm) |
150 ± 10.4 |
149 ± 8.48 |
0.872 |
|
Weight (kg) |
52.4 ± 12.5 |
51.0 ± 9.82 |
0.480 |
|
NYHA classification III/IV, n (%) |
25 (23.1) |
36 (22.0) |
0.934 |
|
Atrial fibrillation, n (%) |
10 (9.26) |
13 (7.93) |
0.870 |
|
Coronary artery disease, n (%) |
19 (17.6) |
33 (20.1) |
0.718 |
|
Peripheral vascular disease, n (%) |
2 (1.85) |
3 (1.83) |
0.654 |
|
LVEF (%) |
57.5 ± 9.61 |
58.5 ± 10.3 |
0.731 |
|
Aortic peak gradient (mmHg) |
89.5 ± 32.3 |
86.5 ± 33.0 |
0.185 |
|
Aortic mean gradient (mmHg) |
50.6 ± 21.2 |
49.8 ± 19.8 |
0.679 |
|
Mitral insufficiency, n (%) |
46 (42.6) |
57 (34.8) |
0.240 |
|
E/Me’ |
25.6 ± 11.9 |
24.5 ± 10.0 |
0.682 |
|
Diabetes, n (%) |
30 (27.8) |
42 (25.6) |
0.798 |
|
Hypertension, n (%) |
89 (82.4) |
140 (85.4) |
0.628 |
|
Dyslipidemia, n (%) |
54 (50.0) |
90 (54.9) |
0.506 |
|
COPD, n (%) |
2 (1.85) |
4 (2.44) |
0.921 |
|
Chronic kidney disease, n (%) |
76 (70.4) |
115 (70.1) |
0.927 |
|
cerebral vascular disease, n (%) |
12 (11.1) |
19 (11.6) |
0.941 |
|
Logistic EuroSCORE |
16.5 ± 13.0 |
16.8 ± 13.4 |
0.879 |
|
STS score |
9.74 ± 5.58 |
7.92 ± 4.91 |
0.229 |
|
Values are presented as the mean ± SD or absolute numbers of patients (%), E: early diastolic left ventricular inflow velocity; Me’: early diastolic velocity of the mitral annulus; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease; Chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73m2; EuroSCORE: European system for cardiac operative risk evaluation; STS: The Society of Thoracic surgery |
|||
Table 1: Preoperative Data Between the fQRS Group and the Non-fQRS Group
Perioperative data for both groups is shown in Table 2. During surgery, 6 patients of the fQRS group and 4 patients of the non- fQRS group needed a temporary pacemaker insertion owing to high-degree atrioventricular block. None of the patients died during the surgery.
|
|
fQRS (n = 108) |
non-fQRS (n = 164) |
p value |
|
Self-expandable valve, n (%) |
29 (26.9) |
37 (22.6) |
0.507 |
|
Surgery time (min) |
79.6 ± 42.7 |
78.3 ± 44.2 |
0.481 |
|
Transfusion (U) |
0.80 ± 1.76 |
0.56 ± 1.39 |
0.128 |
|
Number of catecholamines, n (%) |
0.60 ± 0.81 |
0.59 ± 0.81 |
0.772 |
|
Mild/ moderate/ severe paravalvular leak, n (%) |
41 (38.0) |
77 (47.0) |
0.181 |
|
Need for pacemaker, n (%) |
6 (5.56) |
4 (2.44) |
0.314 |
|
Values are presented as the mean ± SD or absolute numbers of patients (%) |
|||
Table 2: Perioperative Data Between the fQRS Group and the Non-fQRS Group
Table 3 summarized the postoperative complications in this study population. The mortality rates at 2 years were 31.5% (fQRS group) and 3.05% (non-fQRS group). Other postoperative complications, apart from mortality, did not differ between the two groups.
|
|
fQRS (n = 108) |
non-fQRS (n = 164) |
p value |
|
Total mortality, n (%) |
34 (31.5) |
5 (3.05) |
<0.001* |
|
Stroke, n (%) |
9 (8.33) |
20 (12.2) |
0.419 |
|
Permanent pacemaker, n (%) |
6 (5.56) |
8 (4.88) |
0.314 |
|
Vascular complications, n (%) |
2 (1.85) |
4 (2.44) |
0.911 |
|
Postoperative mean aortic gradient (mmHg) |
10.3 ± 4.10 |
10.7 ± 6.21 |
0.769 |
|
Pericardial tamponade, n (%) |
3 (2.78) |
0 (0) |
0.120 |
|
Delirium, n (%) |
2 (1.85) |
3 (1.83) |
0.654 |
|
Heart failure, n (%) |
11 (10.2) |
16 (9.76) |
0.927 |
|
Reintubation, n (%) |
3 (2.78) |
1 (0.61) |
0.348 |
|
Respiratory failure requiring tracheotomy, n (%) |
2 (1.85) |
2 (1.22) |
0.928 |
|
Postoperative days in hospital (days) |
15.3 ± 10.9 |
14.5 ± 8.42 |
0.973 |
|
Rehospitalization for any reaseons, n (%) |
58 (53.7) |
71 (43.3) |
0.119 |
|
Values are presented as the mean ± SD or absolute numbers of patients (%) |
|||
Table 3: Comparison of Clinical Outcomes
The Kaplan–Meier survival curves for two groups are displayed in Figure 3. A Kaplan–Meier survival analysis revealed significantly lower 2-year survival for the fQRS group than for the non-fQRS group (log-rank p < 0.001) (Figure 3). Variables with significant results in univariate analysis for 2-year all-cause mortality included hypertension, dyslipidemia, smoking history, fQRS on ECG, and peripheral vascular diseases. The multivariate analysis identified only one independent predictor for 2-year mortality (hazard ratios, 1.371; 95% confidence interval, 1.072–1.754; p = 0.012) (Table 4). Other than fQRS on ECG before the surgery did not appear to be independent predictors of 2-year mortality. The presence of paravalvular leak, the need for permanent pacemaker and device type were also not independent predictors of 2-year outcome.
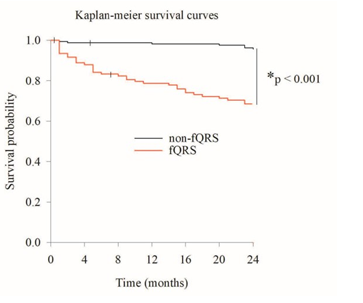
Figure 3: Kaplan–Meier Curves Comparing Outcomes of fQRS Group and Non-fQRS Group Over Two Years (*Log-Rank p < 0.001)
|
Variable |
Number |
Univariate |
Multivariate |
||||
|
Median survival (months) |
p value |
Hazard Ratio |
(95% CI) |
p value |
|||
|
Sex |
|
|
|
|
|
|
|
|
|
Male |
86 |
21.4 ± 0.69 |
0.229 |
|
|
|
|
Female |
186 |
22.1 ± 0.42 |
|
|
|
|
|
|
Hypertension |
|
|
|
|
|
|
|
|
|
Absent |
42 |
21.9 ± 0.39 |
0.040* |
0.923 |
(0.653-1.306) |
0.652 |
|
Present |
230 |
21.0 ± 1.04 |
|
|
|
|
|
|
Dyslipidemia |
|
|
|
|
|
|
|
|
|
Absent |
128 |
21.0 ± 0.62 |
0.040* |
0.936 |
(0.731-1.198) |
0.597 |
|
Present |
144 |
22.8 ± 0.37 |
|
|
|
|
|
|
Diabetes Mellitus |
|
|
|
|
|
|
|
|
|
Absent |
200 |
21.9 ± 0.42 |
0.527 |
|
|
|
|
Present |
72 |
22.1 ± 0.65 |
|
|
|
|
|
|
Smoker |
|
|
|
|
|
|
|
|
|
Absent |
193 |
22.3 ± 0.40 |
0.035* |
1.109 |
(0.849-1.450) |
0.448 |
|
Present |
79 |
21.1 ± 0.73 |
|
|
|
|
|
|
COPD |
|
|
|
|
|
|
|
|
|
Absent |
266 |
21.9 ± 0.36 |
0.880 |
|
|
|
|
Present |
6 |
20.8 ± 3.17 |
|
|
|
|
|
|
fQRS |
|
|
|
|
|
|
|
|
|
Absent |
164 |
23.6 ± 0.21 |
< 0.001* |
1.371 |
(1.072-1.754) |
0.012* |
|
Present |
108 |
19.2 ± 0.79 |
|
|
|
|
|
|
Atrial fibrillation |
|
|
|
|
|
|
|
|
|
Absent |
25 |
22.0 ± 0.37 |
0.411 |
|
|
|
|
Present |
247 |
20.8 ± 1.41 |
|
|
|
|
|
|
Mitral insufficiency |
|
|
|
|
|
|
|
|
|
Absent |
169 |
21.7 ± 0.47 |
0.507 |
|
|
|
|
Present |
103 |
22.2 ± 0.53 |
|
|
|
|
|
|
LVEF |
|
|
|
|
|
|
|
|
|
< 50% |
42 |
21.3 ± 1.05 |
0.923 |
|
|
|
|
> 50% |
230 |
22.0 ± 0.37 |
|
|
|
|
|
|
Coronary artery stenosis |
|
|
|
|
|
|
|
|
|
Absent |
220 |
21.6 ± 0.43 |
0.115 |
|
|
|
|
Present |
52 |
23.0 ± 0.54 |
|
|
|
|
|
|
Peripheral vascular disease |
|
|
|
|
|
|
|
|
|
Absent |
267 |
22.0 ± 0.36 |
0.041* |
1.762 |
(0.647-4.797) |
0.267 |
|
Present |
5 |
19.5 ± 3.30 |
|
|
|
|
|
|
Cerebrovascular disease |
|
|
|
|
|
|
|
|
|
Absent |
241 |
21.9 ± 0.38 |
0.797 |
|
|
|
|
Present |
31 |
22.1 ± 1.01 |
|
|
|
|
|
|
Pacemaker implantation |
|
|
|
|
|
|
|
|
|
Absent |
258 |
21.9 ± 0.37 |
0.847 |
|
|
|
|
Present |
14 |
21.2 ± 1.92 |
|
|
|
|
|
|
Values are presented as the mean ± SD or absolute numbers of patients, CI: confidence interval; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction, *p < 0.05. |
|||||||
Table 4: Univariate and Multivariate Analysis of Potential Predictors for 2 Years Mortality
Discussion
In this study, we evaluated the effect of fragmented QRS on the 2-year mortality of patients with severe aortic stenosis who underwent transfemoral TAVR surgeries. The most important finding of this study was that the presence of fQRS on 12-lead ECG before TAVR procedures was an independent predictor of 2-year mortality, as determined by multivariate analyses. To our knowledge, this was one of the largest studies to evaluate the relationship between 2-year mortality after TAVR and fQRS on preoperative ECG recordings. Fragmented QRS was initially reported as an electrocardiographic indicator characterized by multiple spikes within the QRS complexes, such as RSR’ in the absence of typical bundle branch block pattern [11]. The main pathophysiology for fQRS is related to delayed depolarization caused by electrically inactive fibrotic tissue. Myocardial fibrosis has a negative impact on prognosis in some cardiac pathologies depending on varying degrees of interstitial fibrosis and impaired myocyte ultrastructure [12]. Furthermore, the prognostic significance of fQRS has mainly been documented in patients with coronary artery disease, especially in cases of myocardial infarction, hypertrophic cardiomyopathies and ventricular aneurysm [11,13]. The presence of fQRS in ECG has a significantly higher sensitivity than an abnormal Q wave in detecting myocardial infarction [11]. Previous reports showed a significant correlation between severe AS and presence of fQRS, with up to 46% of AS patients exhibiting fQRS morphology [9,14]. Consistent with these findings, 39.7% of the patients with severe AS in the current study showed fQRS complexes on 12-lead ECG before TAVR. Previously, Acikgoz et al. showed that fQRS could have a significant role in predicting the severity of AS and prognosis [9].
In that study, the presence of fQRS was an independent predictor of ventricular tachyarrhythmia in patients with ischemic or non- ischemic cardiomyopathy. Moreover, another study indicated that fQRS on a standard ECG was a diagnostic marker for arrhythmogenic right ventricular dysplasia - cardiomyopathy [15]. Several studies have shown that fQRS can be used to determine morbidity and mortality involved patients with acute coronary syndrome. Tanriverdi et al. reported that STEMI patients with fQRS showed a higher mortality rate within 48 hours, compared with non-STEMI [16]. The presence of fQRS complexes in patients with non-STEMI was associated with adverse cardiac events such as recurrent myocardial infarction, recurrent angina, and heart failure [17]. In patients with severe AS, the extent of myocardial fibrosis appeared to have a significant effect on the clinical status and long-term survival after aortic valve replacement [18]. Previously, fQRS, related to myocardial fibrosis, was associated with a 1.8-fold increase in long-term outcomes following isolated surgical AVR for severe AS [19]. Additionally, Ay et al. have shown that the presence of fQRS, atrial fibrillation on ECG and low left ventricular ejection fraction (EF < 50%) were independent predictors of death after TAVR [20]. This is consistent with our observation that fQRS on ECG is a useful predictor of mortality after TAVR in patients with severe AS.
The in-hospital mortality and major morbidity rates after cardiac surgery are considered important indicators of quality of care [21]. TAVR is now a well-established therapeutic option in high-risk or inoperable patients with severe symptomatic AS, because it has been demonstrated to be noninferior to SAVR in operable patients [22]. In the large U.K.-transcatheter Aortic Valve Implantation Registry, long-tern outcomes after TAVR were reported with 3 and 5-year survival rates of 61.2% and 45.5%, respectively. In those reports, renal dysfunction, atrial fibrillation, logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE)
> 18.5, respiratory dysfunction, ventricular dysfunction (left ventricular ejection fraction < 30%), and coronary artery disease were identified as independent predictors of mortality [23]. In a single-center study from Germany (1378 patients), type of TAVR access route, residual aortic regurgitation, and concomitant mitral regurgitation were reported to be independent risk factors of a 5-year mortality of 59.1% [24]. In a review of long-term survival rates in TAVR, 83%, 75%, and 65% of the patients were still alive at 1, 2, and 3 years, respectively, after the procedures (31 studies were included, with a total of 13,857 patients) [25]. The 2-year mortality after TAVR procedures in the current study was similar in previous studies. However, the present study identified only fQRS as a strong electrocardiographic predictor of clinical outcome in patients undergoing TAVR surgery. The discrepancies of the results regarding risk factors after TAVR may be related to the differences in patient backgrounds. In our study population, very few patients reported with atrial fibrillation and chronic obstructive pulmonary disease, both of which have been shown to be predictors for mortality after TAVR in many studies. In general, the reported prevalence of atrial fibrillation in Asia was approximately 1%, which was about half of that reported in Western countries [26]. Therefore, the STS and logistic EuroSCORE would also be lower in the current study.
This study had several limitations. First, it was a retrospective study, and our study outcomes were derived from a single university. Owing to the retrospective design of the study, there is a potential for selection bias despite adjusting for estimated confounders. Although the authors adjusted for some possible confounders by Cox regression analysis, the results might still have been influenced by residual unmeasured bias. However, our models are consistent with previous studies. Second, the sample size was relatively small because it was the single-center study. Therefore, further prospective cohort studies with larger sample size may be needed to confirm the findings. Third, we included only patients who underwent transfemoral approach TAVR. The results cannot be generalized to other TAVR approaches in severe AS.
Conclusion
In conclusion, this retrospective analysis of the fQRS found that presence of fQRS on preoperative ECG seems to be a supportive predictor of 2-year mortality of patients following transfemoral TAVR procedures. Future large prospective studies are needed to identify predictors for long-term mortality after TAVR.
Author Contributions
Madoka Yoshimura and Shogo Tsujikawa contributed to the study conception and design. Yuki Takuno, Hideki Hino and Kotaro Hori contributed to data analysis, review and editing. Tadashi Matsuura and Takashi Mori contributed to manuscript preparation. The authors read and approved the final manuscript.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The experimental protocol was established according to the ethical guidelines of Helsinki Declaration and was approved by the Ethics Committee of the Osaka Metropolitan University (approval number: 2021-175).
References
1. Lindman, B. R., Clavel, M. A., Mathieu, P., Iung, B., Lancellotti, P., Otto, C. M., & Pibarot, P. (2016). Calcific aortic stenosis. Nature reviews Disease primers, 2(1), 1-28.
2. Rahhab, Z., El Faquir, N., Tchetche, D., Delgado, V., Kodali, S., Mara Vollema, E., ... & Van Mieghem, N. M. (2020). Expanding the indications for transcatheter aortic valve implantation. Nature Reviews Cardiology, 17(2), 75-84.
3. Carabello, B. A. (1995). The relationship of left ventricular geometry and hypertrophy to left ventricular function in valvular heart disease. The Journal of heart valve disease, 4, S132-8.
4. Everett, R. J., Tastet, L., Clavel, M. A., Chin, C. W., Capoulade, R., Vassiliou, V. S., ... & Dweck, M. R. (2018). Progression of hypertrophy and myocardial fibrosis in aortic stenosis: a multicenter cardiac magnetic resonance study. Circulation: Cardiovascular Imaging, 11(6), e007451.
5. Haukilahti, M. A. E., Eranti, A., Kenttä, T., & Huikuri, H. V. (2016). QRS fragmentation patterns representing myocardial scar need to be separated from benign normal variants: hypotheses and proposal for morphology-based classification. Frontiers in physiology, 7, 653.
6. Kanitsoraphan, C., Rattanawong, P., Mekraksakit, P., Chongsathidkiet, P., Riangwiwat, T., Kanjanahattakij, N..... & Thavaraputta, S. (2019). Baseline fragmented QRS is associated with increased allâ?cause mortality in heart failure with reduced ejection fraction: A systematic review and metaâ? analysis. Annals of Noninvasive Electrocardiology, 24(2), e12597.
7. Kocaman, S. A., Çetin, M., KırıÅ?, T., ErdoÄ?an, T., Çanga, A., DurakoÄ?lugil, M. E., ... & Bostan, M. (2012). The importance of fragmented QRS complexes in prediction of myocardial infarction and reperfusion parameters in patients undergoing primary percutaneous coronary intervention.
8. Gulsen, K., Ince, O., Kum, G., Ozkalayci, F., Sahin, I., & Okuyan, E. (2019). Could fragmented QRS predict mortality in aortic stenosis patients after transcatheter aortic valve replacement? Annals of Noninvasive Electrocardiology, 24(2), e12618.
9. Açıkgöz, E., Yaman, B., Açıkgöz, S. K., Topal, S., Tavil, Y., & Boyacı, N. B. (2015). Fragmented QRS can predict severity of aortic stenosis. Annals of Noninvasive Electrocardiology, 20(1), 37-42.
10. Das, M. K., Saha, C., El Masry, H., Peng, J., Dandamudi, G., Mahenthiran, J., ... & Zipes, D. P. (2007). Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart rhythm, 4(11), 1385-1392.
11. Das, M. K., Khan, B., Jacob, S., Kumar, A., & Mahenthiran,J. (2006). Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation, 113(21), 2495-2501.
12. Azevedo, C. F., Nigri, M., Higuchi, M. L., Pomerantzeff, P. M., Spina, G. S., Sampaio, R. O., ... & Rochitte, C. E. (2010). Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. Journal of the American College of Cardiology, 56(4), 278-287.
13. Reddy, C. V., Cheriparambill, K., Saul, B., Makan, M., Kassotis, J., Kumar, A., & Das, M. K. (2006). Fragmented left sided QRS in absence of bundle branch block: sign of left ventricular aneurysm. Annals of noninvasive electrocardiology, 11(2), 132-138.
14. AÄ?aç, M. T., Korkmaz, L., Bektas, H., Acar, Z., Erkan, H., Kurt, I. H., ... & Çelik, Å?. (2013). Increased frequency of fragmented QRS in patients with severe aortic valve stenosis. Medical Principles and Practice, 23(1), 66-69.
15. Peters, S., Trümmel, M., & Koehler, B. (2008). QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia–cardiomyopathy. Heart Rhythm, 5(10), 1417-1421.
16. Tanriverdi, Z., Colluoglu, T., Unal, B., Dursun, H., & Kaya, . (2018). The prognostic value of the combined use of QRS distortion and fragmented QRS in patients with acute STEMI undergoing primary percutaneous coronary intervention. Journal of electrocardiology, 51(2), 210-217.
17. Li, M., Wang, X., Mi, S. H., Chi, Z., Chen, Q., Zhao, X., &Nie, S. P. (2016). Short-term prognosis of fragmented QRS complex in patients with non-ST elevated acute myocardial infarction. Chinese medical journal, 129(05), 518-522.
18. Milano, A. D., Faggian, G., Dodonov, M., Golia, G., Tomezzoli, A., Bortolotti, U., & Mazzucco, A. (2012). Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. The Journal of thoracic and cardiovascular surgery, 144(4), 830-837.
19. Panc, C., Güler, A., DoÄ?an, A. C., Gülmez, R., Güner, A., & Çelik, Ö. (2022). Fragmented QRS complex may predict long-term mortality after isolated surgical aortic valve replacement in patients with severe aortic stenosis. Interactive cardiovascular and thoracic surgery, 34(1), 26-32.
20. Ay, N. K., Enhos, A., Ay, Y., Bakhshaliyev, N., Nadir, A., Karacop, E., ... & Goktekin, O. (2018). The prognostic value of fragmented QRS in patients undergoing transcatheter aortic valve implantation. Journal of Electrocardiology, 51(6), 923- 927.
21. Ouattara, A., Niculescu, M., Ghazouani, S., Babolian, A., Landi, M., Lecomte, P., ... & Coriat, P. (2004). Predictive performance and variability of the cardiac anesthesia risk evaluation score. Anesthesiology, 100(6), 1405-1410.
22. Vipparthy, S. C., Ravi, V., Avula, S., Kambhatla, S., Mahmood, M., Kabour, A., ... & Mungee, S. (2020). Meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with low surgical risk. The American Journal of Cardiology, 125(3), 459-468.
23. Duncan, A., Ludman, P., Banya, W., Cunningham, D., Marlee, D., Davies, S., ... & Moat, N. (2015). Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the UK Transcatheter Aortic Valve Implantation Registry. JACC: Cardiovascular Interventions, 8(5), 645-653.
24. Zahn, R., Werner, N., Gerckens, U., Linke, A., Sievert, H., Kahlert, P., ... & Schneider, S. (2017). Five-year follow-up after transcatheter aortic valve implantation for symptomatic aortic stenosis. Heart, 103(24), 1970-1976.
25. Chakos, A., Wilson-Smith, A., Arora, S., Nguyen, T. C., Dhoble, A., Tarantini, G., ... & Tian, D. H. (2017). Long term outcomes of transcatheter aortic valve implantation (TAVI): a systematic review of 5-year survival and beyond. Annals of cardiothoracic surgery, 6(5), 432.
26. Tse, H. F., Wang, Y. J., Ai-Abdullah, M. A., Pizarro-Borromeo,A. B., Chiang, C. E., Krittayaphong, R., ... & Hu, D. (2013). Stroke prevention in atrial fibrillation—an Asian stroke perspective. Heart rhythm, 10(7), 1082-1088.



