Research Article - (2025) Volume 2, Issue 1
Experimental Simulation Setup for Reducing the Concentration of Nitrogen Compounds in Livestock Farm Effluents
2Professor, Academician of the Russian Academy of Sciences, Doctor of Technical Sciences, Russia
3Assistant, Russia
4Associate Professor, Candidate of Technical Sciences, Russia
5Assistant, Postgraduate Student, Russian State Agrarian University, Russia
Received Date: Mar 20, 2025 / Accepted Date: Apr 23, 2025 / Published Date: Apr 30, 2025
Copyright: ©©2025 Evgrafov Alexey Vladimirovich, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Vladimirovich, E. A., Nazirovich, D. O., Alexandrovich, F. D., Sergeevich, G. A., Nikolayevich, P. N., et al. (2025). Experimental Simulation Setup for Reducing the Concentration of Nitrogen Compounds in Livestock Farm Effluents. Ann Civ Eng Manag, 2(1), 01-09.
Abstract
In order to reduce the concentration of ammonium in wastewater from livestock farms, the main pollutant for its subsequent application to agricultural fields, a laboratory flow-through electrolysis unit was developed and tested. The experimental setup involved the use of an analog of livestock wastewater, which was constituted by an aqueous solution, designated as the electrolyte. This electrolyte comprised distilled water with the incorporation of ammonium nitrate, at a concentration of 300 mg per liter of NH4 . The objective of the experiment was to study the effect of an electric field on the change of ammonium concentration in the aqueous solution for demineralization of wastewater from livestock farms. Experimental studies were carried out on the developed dynamic electrolysis unit, during which the effect of electric fields on the moving flow of electrolyte simulating wastewater was studied. The electrolyte was passed through electrodes where it was treated by direct electric current with parameters of 12 V and 5 A. The flow rate was 0.45 l/s, the length of the working part was varied during the research and was 0.5 m, 1 m and 1.53 m, respectively. At the end of the experiment, the concentration of nitrogen compounds and hydrogen pH of the electrolyte were determined. It was found that the effect of electric field on NH4 aqueous solution leads to a decrease in the concentration of ammonium nitrogen compounds, at the working part of 0.5 m to 152 mg/l, 1 m - 114 mg/l and 1.5 m - 76 mg/l and a change in the pH of the electrolyte from acidic reaction 5.54 to alkaline - 8.86. Reducing the concentration of ammonium nitrogen reduces the ecological burden on the soil, ground and surface waters and improves the quality of plant products grown on agricultural land where animal husbandry wastewater is used. Changing the pH of the electrolyte from acid to alkaline reaction will have a positive effect on acid soils of the Central Non-Black Earth Zone of the Russian Federation.
Keywords
Livestock Farm Wastewater, Ammonium, Electrolyte, Effluent, Ammonium Compound Concentration Changes, Ammonium Content in Livestock Farm Wastewater
Introduction
Manure with a moisture content of up to 90% is considered semi- liquid, more than 95% is considered liquid, and more than 95% is considered manure effluent [1]. Livestock effluent includes the liquid fraction obtained after separation of manure and manure effluent. The principle scheme of manure separation is shown in Figure 1, which includes a receiving tank where its homogenization and subsequent pumping to the separator, which separates manure into liquid and solid fractions. The liquid fraction enters the storage tank from where it is pumped by pump or gravity to the lagoon.
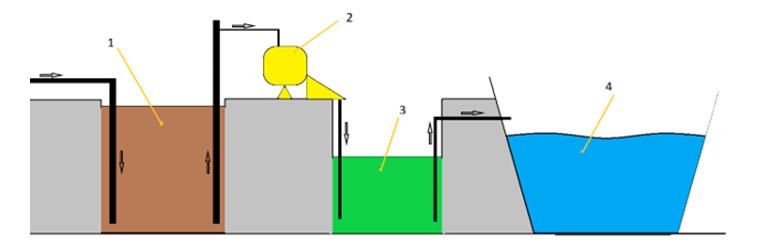
Figure 1: Basic Scheme of Manure Separation into Solid and Liquid Fractions: 1) Receiving Tank, 2) Separator, 3) Storage Tank, 4) Lagoon
Initially, the composition of litter-free manure includes protein at 12.8%, fiber at 19.8%, fat at 2.6%, total nitrogen at 5.2%, phosphorus at 0.07%, along with a substantial amount of minerals and trace elements. The reaction of the manure is weakly alkaline, with the majority of the nitrogen present in an easily soluble ammonia form [1,2]. The composition of livestock effluents is influenced by various factors, including the method of animal housing, the species of livestock, the age of the animals, the composition of the animal rations, and other environmental conditions. A notable aspect of livestock management is the reduction of concentrates in animal diets, a practice that results in a concurrent decrease in nitrogen and phosphorus content and an increase in potassium. The chemical composition of liquid manure, manure effluent, and livestock effluent is predominantly influenced by the degree of dilution with water. Notwithstanding the substantial dilution, these effluents possess notable fertilizing properties. Livestock effluents are regarded as the most intensive pollutants when compared with municipal and industrial wastewater. Nevertheless, these effluents possess notable fertilizing properties. However, in its original form, animal wastewater is not suitable for application as organic fertilizer. The presence of substantial feed residue, veterinary treatment products, and other solid inclusions has been observed, which can lead to pipeline clogging, damage to control valves and other technical components, and the potential presence of pathogenic microorganisms. Consequently, livestock effluent undergoes a series of pretreatment processes prior to application as a fertilizer. These processes include, but are not limited to, separation into solid and liquid fractions, deodorization, disinfection, separation of solids, shredding of feed residues, homogenization, and other operations.
The transition to hydroslush systems for livestock housing has been shown to result in an escalation in the volume of livestock effluents, thereby exacerbating environmental concerns [3,4]. In Russia, where there is a substantial number of livestock farms, this problem is particularly pronounced. For extensive livestock enterprises, the volume of liquid wastewater that must be utilized ranges from 100 to 1,500 cubic meters per day [5]. Conventional methodologies employed for the treatment of livestock wastewater encompass the utilization of anaerobic lagoons, artificial wetlands, and subterranean storage. However, these conventional approaches are characterized by their prolonged operation time, substantial financial expenditure, and the necessity for extensive treatment areas, often resulting in the generation of voluminous sludge. Consequently, electrolysis has emerged as a promising alternative, offering versatility, simplicity, and environmental compatibility. However, to date, scientists worldwide have offered their views on the technical parameters of electrolysis plants [6]. The objective of this study is to examine the impact of an electric field on the variation in ammonium concentration within an aqueous solution during its movement within an experimental unit. This investigation aims to ascertain the necessary parameters of a device for the detoxification of wastewater from livestock farms.
Materials and Methods
Study Area
The wastewater from livestock complexes, with a volume of 1 m3, contains 0.8–1.4 kg of nitrogen, 0.3–0.7 kg of phosphorus, and 0.4–1.2 kg of potassium. The mineral composition of nitrogen is predominantly present in the form of ammonium, with concentrations in wastewater that exceed those of phosphorus and potassium mineral compounds. Notably, livestock wastewater can contribute to the nitrogen content of soil, with total nitrogen comprising 3.2% of the total volume, with more than 50% of nitrogen present in the readily soluble form of ammonia. The approximate composition of wastewater from livestock complexes is presented in the following table. It should be noted that the composition may vary depending on the method of animal housing, livestock, age, type, and feeding rations.
|
Wastewater |
pH |
K, mg/l |
NH4, mg/l |
P2O5, mg/l |
Suspended sludge, mg/l |
|
Wastewater from pig farms |
7.5 |
274 |
573 |
152 |
1347 |
|
Wastewater from cattle |
7.4 |
860 |
420 |
158 |
1347 |
Table: Wastewater Composition of Livestock Farms
While excess nitrogen can accumulate in plants without causing harm, an excess of this element poses a significant threat to human and animal health. The application of elevated nitrogen doses has been demonstrated to result in a decline in crop yield, a disruption of the sugar-protein and calcium-phosphorus ratios, and a substantial increase in nitrate content. The predominant forms of mineral nitrogen in soil include ammonium, nitrates, and nitrites. The latter, while present in negligible amounts, are not absorbed by the soil-absorbing complex [7]. In soil, ammonium undergoes partial nitrification, a process facilitated by microorganisms and characterized by an oxidative mechanism. Two categories of wastewater treatment and utilization methods have been identified. The first group, known as the "regenerative group," involves the extraction of substances from wastewater for subsequent reuse. A notable example is wastewater from gas-generating plants, which contains phenols and vinegar. These substances can be extracted and reused [8]. The second and most prevalent group is the destructive group, which involves the degradation of pollutants through reduction or oxidation processes, followed by the removal of the degradation products. These methods encompass a range of processes, including pretreatment and discharge to water bodies, utilization as a nutrient source in agricultural settings, incorporation into filtration fields for additional treatment, and the treatment of effluents in biological-oxidizing (BOC) ponds. Beyond these applications, effluents from livestock farms can be valorized through diverse processes, including: the production of yeast raw materials and protein additives; processing in biogas plants to generate methane; evaporation; hydroponic cultivation of green vitamin feeds; disinfection in algae (BOKS), crayfish, and fishpond systems; biological lagoons; disinfection by ozonation in specialized installations with the aid of hydroelectric shock; utilization as organic fertilizers or composts; and transportation to agricultural fields, among others [9].
The operational data from full artificial biological treatment facilities indicates that the efficiency of treatment is low, with rates ranging from 50% to 60% [10]. During the process of passing livestock effluents through treatment facilities, there is a redistribution of biogenic elements in by-products, with losses amounting to 24% nitrogen, 2.6% phosphorus, and 7.2% potassium. Furthermore, the solid fraction, which is formed during the separation of wastewater into fractions prior to its introduction into treatment facilities, and activated sludge sediment, necessitate additional treatment in soil. The most environmentally safe and economically viable method of utilizing wastewater from livestock farms is to apply it to agricultural fields during the cultivation of crops. Typically, these materials are introduced into the soil through tilling or irrigation methods. This approach ensures the removal of a substantial proportion of the constituents present in wastewater, concomitant with the harvest of the crop. However, stringent monitoring procedures must be implemented to ensure the quality of the produce, particularly with regard to the nitrogen content [11,12]. In order to reduce the ammonium content in livestock effluents for application to agricultural fields, it is necessary to develop more effective methods of their preparation. This is important to prevent or minimize pollution of agricultural landscapes and water bodies as a result of economic activity of livestock enterprises of the agro-industrial complex. The operation of these enterprises generates large volumes of manure and manure runoff, which have a significant impact on the ecological state of the environment. The high fertilizing value of livestock effluents should be leveraged to restore and maintain soil fertility, thereby reducing economic costs associated with mineral fertilizers. Concomitantly, adherence to environmental norms concerning their safe usage is imperative. Pollution is defined as the introduction of techno genesis products into the environment that have a deleterious effect on humans and other living organisms, biological components, and that engender negative toxic ecological consequences [13]. It is important to note that not all pollutants are equally neutralized by the environment; some are redistributed among the various components of nature.
Among the substances contained in liquid effluents after their separation into solid and liquid phases, nitrogen compounds stand out as the most mobile and hazardous from an environmental safety perspective. Nitrates (NO3) and ammonium nitrogen (NH4) are particularly noteworthy. The greatest quantity of nitrogen compounds is absorbed by vegetation, as plants are capable of assimilating 200 to 600 kilograms of pure nitrogen per hectare of crops annually, contingent on the prevailing climatic zone. An excess of nitrogen can compromise the quality of crops and even pose a threat to human health. Consequently, the cultivation of crops intended for direct human consumption in wastewater disposal systems is not recommended to mitigate potential health hazards. It is noteworthy that livestock effluent storage in lagoons can result in nitrogen compound losses ranging from 20% to 30%, contributing to atmospheric air pollution [14]. Inorganic nitrogen in soil is predominantly present as ammonium, nitrates, and nitrites.
The latter are present in negligible amounts. Ammonium in soil is present in the soil solution in the ionic form NH4 +, as well as in the soil-absorbing complex in the exchange and non-exchange- absorbed state, easily replacing hydrogen, potassium, and partially calcium ions. Ammonium is predominantly absorbed by clay ternary minerals, representing the final form of NH4 absorption, while dissolved and exchange-absorbed states serve as intermediate forms. The fixation process is reversible. The process of ammonium transfer from its fixed position is facilitated by the activities of plants and microorganisms, which extract NH4 + from the soil solution and facilitate its exchange-absorbed state. This results in a shift in the dynamic equilibrium reaction, NH4 + (solution) NH + (exchangeable) NH + (fixed) [1,8]. Ammonium in soil undergoes nitrification, an oxidative process, by microorganisms. The initial step in this process is the oxidation of NH4 to NO2, which is an unstable form. Subsequent to this, NO2 is converted to NO3, a form that is not absorbed by the soil. The denitrification process, defined as the reduction of nitrate nitrogen to gaseous forms (NO, N2O, N2), occurs in anaerobic conditions and an alkaline reaction of the medium. This process also takes place in the soil. The magnitude of gaseous nitrogen losses from soil has been estimated to range from 15% to 20%. The nitrogen content of soil is also influenced to a great extent by cultivated crops. The ratio of N-NO3: The exchangeable nitrogen ratio (N-NH4) is 1:0.1-0.04 for row crops, 1:0.1-0.15 for cereals, and 1:0.25-0.35 for perennials [8]. Nitrogen (N) is an essential element for plant life, found in proteins and nucleic acids (e.g., RNA, DNA), which play a crucial role in metabolism. Chlorophyll, enzymes, and other nitrogen compounds (e.g., nitrates, nitrites, ammonia) are also found in plants. An unlimited supply of nitrogen to plants results in the inability to process it into complex organic substances, leading to its accumulation as nitrates. This, in turn, alters the ratio of substances within plants [7-10]. Excessive application of runoff has been demonstrated to result in nitrates leaching into the underlying soil, leading to an increase in the concentration of ammonium in the soil solution. This, in turn, facilitates the leaching out of the soil, which contributes to groundwater contamination by downward- flowing moisture.
Design and Construction of the Experimental Unit
The design and operating conditions of the electrolyzer are pivotal in determining the efficiency of contaminant removal from animal waste streams, as evidenced by previous studies. Despite the utilization of a uniform electrode material, disparities in design and operating parameters give rise to substantial variations in process performance. In the context of swine wastewater, the majority of studies have employed laboratory batch reactors, while continuous operations have predominantly been executed in conjunction with other treatment technologies [15,16]. The process of water purification by electrolysis is predominantly executed in electrolyzers that are equipped with vertically mounted electrodes, frequently arranged in a configuration resembling a block of rectangular plates with a thickness ranging from 5 to 10 millimeters. Aluminum and its alloys, as well as iron, are predominantly utilized in the fabrication of anodes and cathodes. Anodic dissolution of the electrode material has been shown to result in clogging of the treated effluent with metal hydroxides. This phenomenon is undesirable due to the potential for such materials to infiltrate the soil. These compounds are indicative of a sludge with a non-dense structure, which necessitates the implementation of supplementary treatment devices, such as the electrolyzer- sump, galvanocoagulator-electrolyzer, and electrolyzer-flotator. The proposed design is intended to address the aforementioned issues and enhance the efficiency of wastewater treatment in livestock complexes through electrolysis, obviating the need for additional treatment apparatuses. To conduct the studies, a dynamic electrolysis unit was designed and assembled, including the following main elements: mounting frame; electrolyte tank; direct current source; working part (electrolyzer) 0.5 m long, with the outer electrode (cathode) made of aluminum alloy and the inner electrode (anode) made of graphite; current carrying wires; flow control tap; flow meter; discharge hose (Figures 2, 3, 4,5).
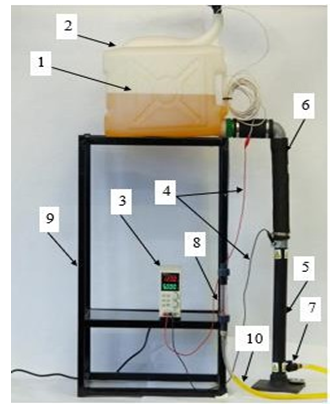
Figure 2: Photo of Dynamic Electrolysis Unit Where; 1) Electrolyte Level, 2) Tank, 3) Direct Current Source, 4) Current Carrying Wires, 5) Working Part (Electrolyzer), 6) Flexible Hose, 7) Faucet, 8) Flow Meter, 9) Mounting Frame, 10) Discharge Hose
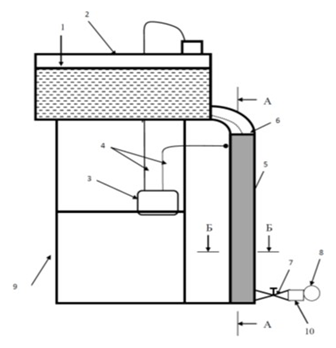
Figure 3: Scheme of laboratory flowing electrolysis unit where; 1) Electrolyte Level, 2) Tank, 3) Constant Current Source, 4) Current Carrying Wires, 5) Working Part (Electrolyzer), 6) Flexible Hose, 7) Faucet, 8) Flow Meter, 9) Mounting Frame, 10) Discharge Hose
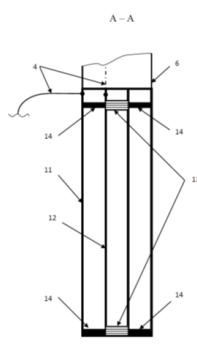
Figure 4: Longitudinal Section A - A of the Electrolyzer where: 4) Current Carrying Wires, 6) Flexible Hose, 11) External Electrode in the Form of a Hollow Cylinder, 12) Internal Electrode in the Form of a Hollow Cylinder, 13) Hermetic Plugs on the Internal Electrode, 14) Holding Spacers
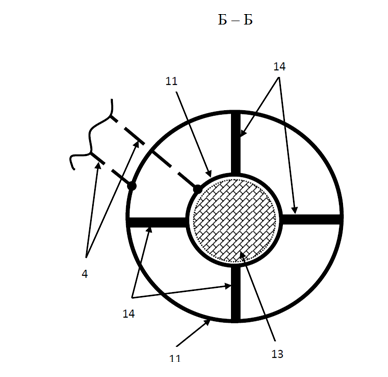
Figure 5: Cross Section B - B of the Electrolyzer where: 4) Current Carrying Wires, 11) External Electrode in the Form of a Hollow Cylinder, 12) Internal Electrode in the Form of a Hollow Cylinder, 13) Hermetic Plug on the Internal Electrode 14) Holding Spacers
The following characteristics are exhibited by the electrolyzer: parameters of the inner electrode (+anode):
Diameter of the contact surface dinside = 0.016 m; - length of the circle linside= 0.05 m; - cross-sectional area dinside= 0.0001 m2.
The parameters of the external electrode, designated as the cathode, are as follows:
Diameter of the contact surface doutside=0,046 m;
Length of the circle loutside=0,144 m;
Cross-sectional area doutside= 0,0003 m2.
It was established through experimental means that when employing graphite as an anode in the electrolysis of a NH3NH4 solution, the formation of aluminum hydroxide Al (OH3) is excluded, in contrast to the use of aluminum alloy for this element. The electrolyte, containing an initial NH3NH4 concentration of 300 milligrams per liter and a pH of 5.54, was introduced into a dynamic electrolysis unit and passed through the working section at flow rates of 0.15, 0.30, and 0.45 liters per second. Subsequent to the treatment, the final ammonium concentration and hydrogen pH were ascertained. The design presented in the article is experimental and is intended to develop a methodology for determining the parameters of industrial plants of this type, so the flow rates were set in increments of 0.15 l/sec for the possibility of determining the dependence of NH3NH4 concentration change on the flow rate, geometric parameters of electrodes for designing a real sample for specific livestock complexes, in addition, when the temperature of livestock effluent in the lagoon decreases, it is possible to reduce the flow rate in the electrolyzer to increase their temperature.
Result and Discussion
The treatment of the electrolyte with initial parameters NH3NH4 300 mg/l and pH 5.54 by electric fields during its movement through the working part of the dynamic electrolysis unit led to a decrease in the ammonium nitrogen content and a change in the hydrogen index. The following decreases in concentration were observed: at a flow rate of 0.15 liters per second (l/sec), 54 milligrams per liter (mg/l), or 82% of the initial value; at 0.30 l/ sec, 104 mg/l, or 65.3%; at 0.45 l/sec, 153 mg/l, or 49% (Figure 6). As indicated in the preceding static experimental studies, a change in the hydrogen index was observed in a non-flowing unit [17]. 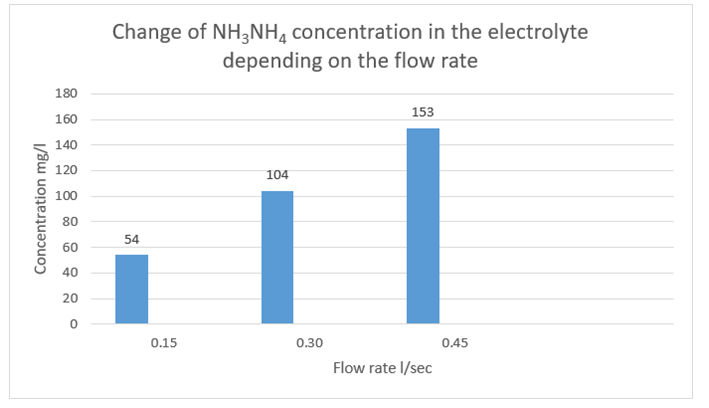
Figure 6: Change in Electrolyte Concentration as a Function of Flow Rate at an Initial Value of 300 mg/l NH3NH4
The detoxifying potential of electrochemical treatment has been demonstrated in previous studies [3]. During the detoxification of wastewater from pig farms, the efficiency of NH4 + removal exhibited a range of 87%, contingent upon the operational conditions [18]. It has been established that an increase in ionic strength results in an increase in current density at a constant cell voltage. Conversely, an increase in incoming conductivity at a constant current density results in a decrease in cell voltage [19]. The incoming conductivity in the purification process is predominantly influenced by the type and concentration of electrolytes. During the purification process, various salts, including sodium chloride (NaCl), barium chloride (BaCl2), potassium chloride (KCl), sodium sulfate (Na2SO4), and potassium iodide (KI), have been utilized as electrolytes. Notably, sodium chloride (NaCl) is the most prevalent electrolyte employed to enhance electrical conductivity. The incorporation of NaCl in the purification process provides a substantial amount of Cl-, which subsequently produces Cl at the anode and instantaneously forms HOCl through a reaction with water. The HOCl, in turn, reacts with the ammonia (NH3) present in the solution, leading to the production of nitrogen (N2) gas. Furthermore, the presence of NaCl has been shown to mitigate the adverse effects of other anions, such as bicarbonate (HCO ) and sulfate (SO 2-), which xhave been observed to diminish current efficiency by promoting the precipitation of calcium (Ca2+) and magnesium (Mg2+) ions and the formation of an oxide layer [20]. Holt et al. recommended that for effective and efficient electrochemical wastewater treatment, the Cl- concentration should be maintained well above 200 mg/l (0.02%) [21]. Furthermore, the generation of chloride (Cl-) during electrochemical treatment has been demonstrated to play a pivotal role in the process of water disinfection. However, during the experimental studies, the addition of these salts to the electrolyte was not incorporated. Temperature is another process parameter that regulates the pollutant removal efficiency of electrochemical treatment of waste streams [22]. However, to the best of the authors' knowledge, no studies have been conducted that have investigated the effect of temperature on the removal of pollutants in the electrolysis of animal wastewater. Typically, the electrolytic treatment of livestock wastewater is conducted at ambient temperature (18 to 27 °C) [23-25]. In this experimental study, the solution temperature was set at 22°C, a choice that aligns with the findings of other researchers.
The initial pH of the incoming stream is a pivotal operating parameter that influences the efficacy of electrochemical wastewater treatment. The pH level affects the ionic conductivity of wastewater, as well as the dissolution of electrodes and the formation of hydroxyl radicals. During the electrolysis of animal wastewater using sacrificial electrodes, the removal process of solids and dissolved organic matter can be explained by the latter mechanism [26,27]. Consequently, effective electrochemical treatment of livestock wastewater can be carried out in the pH range of 6-8 [28]. However, it is imperative to note that the efficacy of this range may vary depending on the characteristics of the wastewater and the targeted pollutant. Consequently, experimental optimization of this range is necessary to ensure the optimal treatment of specific wastewater samples. The study also documented a variation in the hydrogen index of the electrolyte, which was contingent on the flow rate configured in the electrolyzer. At the inception of the experiment, the flow rate was set at 5.54, and the subsequent fluctuations are delineated in Figure 7. At a flow rate of 0.15 L/sec, the hydrogen index registered at 8.88. At flow rates of 0.30 L/sec and 0.45 L/sec, the hydrogen index values were 7.77 and 6.47, respectively. This phenomenon can be attributed to the alterations in the concentration of dissolved substances within the electrolyte during the electrolysis process, resulting in a corresponding change in the hydrogen index.
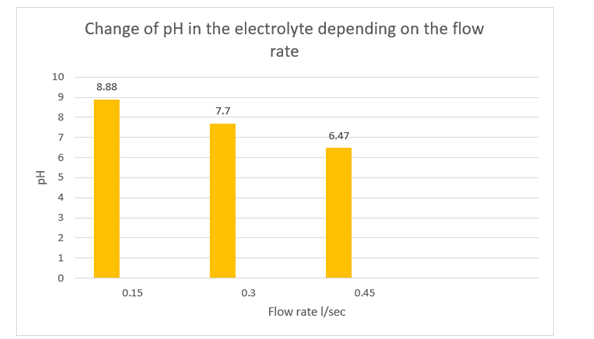
Figure 7: Variation of Electrolyte pH as a Function of Treatment Time at Initial pH Values of 5.53 and Concentration of 300 mg/l NH3NH4
Ammonium losses from the electrolyte were attributed to the release of ammonia at the cathode, specifically the formation of NH3. According to extant literature, the concentration of ammonia in the air at livestock complexes can reach from 35 to 98 milligrams per cubic meter (mg/m3) due to microbiological activity. This exceeds the maximum permissible level (MPL) from 1.5 to 5.0 times. The MAC is 0.2 mg/m3 (GN 2.1.6 3492 - 17) [13,14]. The collection of NH3 in the air of buildings and premises through the use of scrubbers is not a viable option in the area of lagoons, which are located in the open territory. This is due to the fact that NH3 in the air is a prerequisite for air pollution and a direct threat to the health of personnel of livestock complexes. The proposed prototype of the dynamic unit facilitates the separation of ammonia from lagoons containing livestock effluents, concentrating it within a single enclosed space. This allows for subsequent destruction of the ammonia via a scrubber, thereby addressing the issue of soil, groundwater, and surface water contamination. Additionally, it enhances the quality of crop production cultivated on fields utilized for the application of animal by-products (APP). It is noteworthy that in areas where APP is employed, fodder crops are predominantly cultivated, thereby impacting the quality of meat and dairy products.
Conclusion
The findings of the research indicate that the most optimal configuration for the electrolysis unit is the implementation of electrodes composed of the following materials: an aluminum alloy anode and a graphite cathode. This combination eliminates the formation of metal hydroxides, which, when introduced into the soil during the application of effluents in agricultural fields, can adversely affect soil quality and crop yield. It is noteworthy that the anode composed of graphite exhibits a prolonged service life when compared with aluminum alloys. Furthermore, the byproducts of its degradation are not hazardous to the soil or agricultural products. The efficacy of the developed design for the removal of ammonium from the effluents of livestock complexes has been demonstrated by experimental laboratory studies. The obtained results will facilitate the development of a calculation method for industrial installations of this type in livestock complexes. This method will depend on the concentration of nitrogen compounds in each specific case. The composition of effluents will be contingent on housing, feeding, age, and breed of animals, as well as the technological schemes used for the removal and utilization of manure.
References
1. Shevchenko, V. A., Soloviev, A. M., Bondareva, G. I., Popova, N. P., Menshikova, S. A., Evgrafov, A. V., ... & Sheshenev, N. V. (2021). Safe use of organic waste from pig-breeding complexes when involving agricultural lands in circulation.
2. Gubin, V. K., Shevchenko, V. A., & Kudryavtseva, L. V. (2024). Reclamation Systems for Growing Shelterbelts on Saline Soils Under Saltwater Irrigation. Power Technology and Engineering, 58(2), 267-269.
3. Methodology for ensuring environmentally safe utilization of livestock effluents on agricultural irrigation fields / O. N. Didmanidze, A. V. Evgrafov, N. N. Pulyaev [et al.]. - Moscow: OOO “Sam Polygraphist”, 2024. - 164 Ñ. - ISBN 978-5-00227-323-2.
4. Evgrafov, A. V., Pulyaev, N. N., & Filimonov, D. A. (2024). Results of experimental studies of the influence of electric fields on the ammonium content in livestock wastewater. Agroinzheneriya, 24(5), 4-9.
5. Tolkachev, G. Yu. Principles of monitoring of water bodies to study processes and phenomena in the restoration of soil fertility and reclamation of degraded reclaimed land / G. Yu. Tolkachev, B. I. Korzhenevsky, N. V. Kolomiitsev // Life Safety. - 2022. - â?? 7(259). - С. 37-43.
6. Khanaum, Mustari & Borhan,. (2023). Electrocoagulation: An Overview of the Technology for Livestock Farm Wastewater Treatment. 11. 1-16.
7. Danilina, E. I., Rogulin, V. V., & Babicheva, E. A. (1996). Determination of exchangeable and water-soluble ammonium in soils by modified indophenol methods. Bulletin of Chelyabinsk State University, 4(1), 28-39.
8. Ismaylova, S. G., Damirova, K. I., & Rustamova, E. E. (2022). INFLUENCE OF ORGANIC FERTILIZERS ON NITROGEN CONTENT IN VEGETABLE CROPS. In ENVIRONMENTAL PROTECTION-THE BASIS OF COUNTRY SECURITY (pp. 243-245).
9. Boytsova, L. V., Zinchuk, E. G., & Neprimerova, S. V. (2018). The influence of different types of land use on the accumulation of organic matter in sod-podzolic soils. Problems of Agrochemistry and Ecology, 3, 45-50.
10. Khalmanov, N. T. (2014). THE INFLUENCE OF SIDERATION ON CHEMICAL PROPERTIES OF SIEROZEM SOILS OF THE ZARAFSHAN VALLEY IN THE REPUBLIC OF UZBEKISTAN. The Way of Science, 46-48.
11. Khadanovich, A. V., & Zaitseva, A. D. (2020). The nature of nitrate ion accumulation in the soil-plant system. Epoch of Science, (21), 92-96.
12. Khadanovich, A. V., & Denisenko, Ya. A. (2020). INFLUENCE OF HYDRO-METEOROLOGICAL FACTORS ON THE ACCUMULATION OF NITRATE IONS IN PLANTS GROWN UNDER MICRO-FIELD EXPERIMENT CONDITIONS. Current Scientific Research in the Modern World, (1-7), 147-152.
13. Krivolapov, I. P., Koldin, M. S., & Shcherbakov, S. Yu. (2016). Study of the efficiency of air purification in livestock complexes from ammonia and hydrogen sulfide. Technologies of food and processing industry of the agro-industrial complex – healthy food products , (3 (11)), 9-18.
14. Galinger, I. O. (2023). Environmental problems of pig farming and ways to solve them. In Problems of agroecology of the agro-industrial complex of Siberia (pp. 175-179).
15. Mores R, Treichel H, Zakrzevski CA et al (2016) Remove of phosphorous and turbidity of swine wastewater using electrocoagulation under continuous flow. Sep Purif Technol 171:112-117.
16. Wang F, Wei J, Zou X et al (2019) Enhanced electrochemical phosphate recovery from livestock wastewater by adjusting pH with plant ash. J Environ Manage 250:109473.
17. Evgrafov, A., Filimonov, D., & Perevozchikova, N. (2025). Experimental studies on the effect of electric field on ammonium nitrogen compounds in livestock wastewater. In E3S Web of Conferences (Vol. 613, p. 05009). EDP Sciences.
18. Chen R-F, Liu T, Rong H-W et al (2021a) Effect of organic substances on nutrients recovery by struvite electrochemical precipitation from synthetic anaerobically treated swine wastewater. Membranes 11:594.
19. Kobya, M., Can, O. T., & Bayramoglu, M. (2003). Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. Journal of hazardous materials, 100(1- 3), 163-178.
20. Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11-41.
21. Holt, P. K., Barton, G. W., & Mitchell, C. A. (2005). The future for electrocoagulation as a localised water treatment technology. Chemosphere, 59(3), 355-367.
22. Sahu O, Mazumdar B, Chaudhari PK (2014) Treatment of wastewater by electrocoagulation: a review. Environ Sci Pollut Res 21:23972413.
23. Ghahremani, H., Bagheri, S., Hassani, S. M., & Khoshchehreh, M. R. (2012). Treatment of dairy industry wastewater using an electrocoagulation process. Advances in Environmental Biology, 6(7), 1897-1901.
24. Benaissa, F., Kermet-Said, H., & Moulai-Mostefa, N. (2016). Optimization and kinetic modeling of electrocoagulation treatment of dairy wastewater. Desalination and Water Treatment, 57(13), 5988-5994.
25. Huang, K. L., Wei, K. C., Chen, M. H., & Ma, C. Y. (2018). Removal of organic and ammonium nitrogen pollutants in swine wastewater using electrochemical advanced oxidation. International Journal of Electrochemical Science, 13(12), 11418-11431.
26. Jiménez, C., Sáez, C., Martínez, F., Cañizares, P., & Rodrigo, M. A. (2012). Electrochemical dosing of iron and aluminum in continuous processes: a key step to explain electro-coagulation processes. Separation and purification technology, 98, 102-108. https://doi.org/10.1016/j.seppur.2012.07.005
27. Marriaga-Cabrales, N., & Machuca-Martínez, F. (2014). Fundamentals of electrocoagulation. Evaluation of electrochemical reactors as a new way to environmental protection, 661, 1-16.
28. Tak, B. Y., Tak, B. S., Kim, Y. J., Park, Y. J., Yoon, Y. H., & Min, G. H. (2015). Optimization of color and COD removal from livestock wastewater by electrocoagulation process: application of Box–Behnken design (BBD). Journal of industrial and engineering chemistry, 28, 307-315.